Therapeutic Vitrectomy
Since the original report of combined pars plana vitrectomy and lensectomy for complicated cataract in 1978,1 numerous reports describing the therapeutic benefit of vitrectomy in the management uveitis and its complications have appeared in the literature. No prospective, controlled clinical trail, however, has been conducted to critically evaluate the role of PPV as a single or combined procedure for this indication. A superb review of the literature and discussion of this subject by Drs. Matthias Becker and Janet Davis has recently been published.2 Based on this survey, visual acuity was stated as improved in 708 eyes (68 percent), unchanged in 202 eyes (20 percent) and worsened in 124 eyes (12 percent) despite the confounding co-morbidities of cystoid macular edema and cataract and the frequent need for additional surgical procedures. Intermediate uveitis was present in nearly half of these eyes.
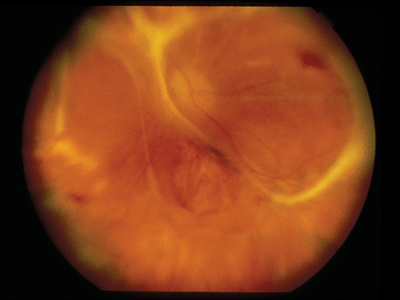
Figure 1. Combined tractional/rhegmatogenous retinal detachment
in a patient with acute retinal necrosis syndrome.
Indications
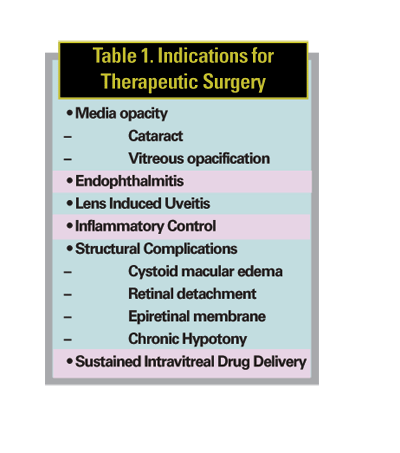
Indications for therapeutic vitrectomy in uveitis have included the following: 1) significant media opacity (cataract, inflammatory or hemorrhagic vitreous opacification) causing visual loss or precluding adequate view of the posterior segment; 2) endophthalmitis or lens-induced uveitis; 3) inflammatory control; 4) structural complications of uveitis including, CME unresponsive to medical treatment, retinal detachment, epiretinal membrane (ERM), and chronic hypotony; 5) and most recently, sustained intravitreal drug delivery (See Table 1).
Perioperative Management
Inconsistent data exist as to the precise role of perioperative immunosuppressive therapy in eyes undergoing vitreoretinal surgery for uveitis. While complete inflammatory quiescence may not be attainable or realistic in some patients requiring urgent care, the experience from cataract surgery in uveitis suggests that vigorous efforts to establish a definitive diagnosis and aggressive control of perioperative inflammation are essential both for successful surgical outcomes and to reduce the risk of postoperative complications. For non-emergent procedures, it is certainly advisable to adequately suppress inflammatory activity utilizing topical, regional and systemic steroids with or without systemic steroid-sparing immunomodulatory therapy as necessary for a minimum of three months prior to surgery. This is particularly important if cataract and intraocular lens implantation is contemplated.
Preoperative ancillary assessment might include B-scan ultrasonography or ultrasonic biomicroscopy in the presence of media opacity or hypotony to detect underlying chorioretinal pathology, such as and exudative retinal or choroidal detachment, and cyclitic membranes, which might influence the surgical plan. Fluorescein angiography may disclose retinal ischemia or neovascularization and serve as a surrogate marker for inflammatory activity in the postoperative period following vitrectomy. Optical coherence tomography is helpful in confirming the presence of epiretinal membranes and in distinguishing inflammatory macular edema from that due to vitreomacular traction, thereby identifying cases that are more likely to respond to surgical intervention.
Media Opacity
Various surgical techniques have been proposed for the management of uveitic cataracts. Among them are pars pars plana lensectomy, and extracapsular cataract extraction (phacoemusification) with or without IOL implantation, in combination with PPV, especially in the presence of significant vitreous opacification or underlying vitreoretinal pathology. Pars plana lensectomy/vitrectomy with complete removal of the posterior capsule remains the preferred technique in selected chronic uveitic entities, such as juvenile idiopathic arthritis-associated iridocylcitis,3 and in phacolytic uveitis. In other cases, a rim of anterior capsule may be retained should secondary placement of a ciliary sulcus posteior IOL be anticipated. Combined phacoemulsification with IOL implantation and PPV as a single procedure may be appropriate in selected patients with cataract and pathologic vitreous whose disease has been well-controlled preoperatively or is in remission as was reported recently, with improved visual acuity in 76 percent of 36 eyes with chronic uveitis.4 While IOL placement in the ciliary sulcus may theoretically reduce the incidence of postoperative synechiae, it may promote chronic flare and CME due to mechanical irritation of the iris and ciliary body.5,6 For this reason, in-the-bag placement is widely recommended. Traditionally, large, one-piece, all-PMMA lenses had been advocated. Recent studies suggest, however, that hydrophobic acrylic IOLs are associated with favorable outcomes, good capsular biocompatibility and lower rates of uveitis recurrence as compared to silicone IOLs.7,8 Progressive, vitreous opacification due to chronic inflammation, despite adequate treatment, or due to non-clearing vitreous hemorrhage may be visually significant, amblyogenic, or obscure underlying vitreoretinal pathology and require PPV. Uveitic entities frequently associated with retinal neovascularization and vitreous hemorrhage include pars planitis, ocular sarcoidosis, idiopathic retinal vasculitis (Eales' disease), Adamantiades-Behçet's disease, and systemic lupus erythematosus. In addition to clearing the visual axis and addressing co-existent vitreoretinal pathology, the surgical goals also include treatment of underlying retinal ischemia or pars plana neovascularization with ablative laser photocoagulation.
Inflammatory Control
While essential in the management of endophthalmitis and lens-induced uveitis, PPV may also modulate the natural history of inflammation in patients with intermediate or diffuse uveitis. It has been hypothesized, but not proven, that the permanent removal of antigenic load and pro-inflammatory mediators through vitrectomy affects a change in the immunologic milieu of the eye that promotes inflammatory control and facilitates the efficacy of anti-inflammatory therapy.
Among the uncontrolled reports for which data are available, especially in the context of intermediate uveitis, a reduction in systemic medical therapy was noted in more than half the cases with a nearly uniform clearing of the vitreous following PPV.2 Specifically, many investigators have reported a reduction in the frequency and severity of inflammatory recurrences,1, 9-19 while others conclude that the course of long-standing uveitis is not influenced by vitrectomy.20,21 It is possible that vitreous inflammation is a secondary manifestation of the underlying immunopathology of autoimmune uveitis, where antigen-specific T-cells are activated extraocularly (in the spleen and lymph nodes) with subsequently homing to the eye, and that vitreous removal creates the illusion of quiescence while "sub-clinical" intraocular inflammation persists unabated.
Other surrogate markers for inflammatory activity, such as vascular leakage on fluorescein angiography or abnormalities detected with electroretinography, may be useful in this regard. For these reasons, it is not clear if PPV should precede systemic therapy as proposed by Dr. Henry Kaplan26 in his therapeutic step-ladder algorithm for the management of intermediate uveitis or whether all patients should have preoperative IMT as advocated by Dr. Stephen Foster.23
Structural Complications
PPV in uveitis often requires additional vitreoretinal procedures, such as separation of the posterior hyaloid and membrane peeling, retinal detachment repair, laser photocoagulation and/or cryotherapy to address structural complications.
• CME. CME refractory to medical therapy is a relative indication for PPV, especially in the presence of another structural complication, such as ERM or vitreomacular traction seen clinically or on OCT. Case selection is important, as chronic CME is less likely to respond to surgery due to the presence of permanent architectural damage. This damage is suggested by the presence of fixed cysts clinically, widening of the foveal avascular zone on fluorescein angiography, and attenuation or the central retinal thickness on OCT. Three studies have been undertaken that specifically address the effect of PPV on recalcitrant CME in uveitis.24-26 Among one group of nine patients undergoing PPV without membrane peeling,24 seven of 11 eyes had an improvement of four or more lines of Snellen acuity within four weeks. CME improved by clinical examination and on fluorescein angiography in nine eyes and by clinical examination alone in two eyes. Of the six eves with a diagnosis of pars planitis, only three were among those noted to improve. Dr. Junichi Kiryu and associates reported an improvement in visual acuity in 56 percent and clinical resolution of CME in 78 percent of 18 eyes of 14 patients with sarcoid uveitis who underwent PPV for medically unresponsive CME.25 Membrane peeling was performed in half of these patients, while remainder had posterior vitreous detachments without ERM formation. Of note, half of these patients required supplemental systemic steroids, periocular steroids or acetazolamide postoperatively to resolve their macular edema.
Another group performed PPV for CME in 42 eyes of 32 patients with intermediate uveitis with resolution of CME in 59 percent and improvement in visual acuity in 50 percent.26 In the previously mentioned survey of the literature, Drs. Becker and Davis calculated that the median reported percentage of patients per study with CME decreased from 36 percent preoperatively to 18 percent postoperatively.2 It is likely, however, that with the expanding indications for the use of intravitreal steroids in the treatment of CME in general, and in uveitis in particular,27-29 PPV may become relegated to the final interventional measure in the management of this complication.
• ERM. Epiretinal membranes are common in eyes with uveitis, and when not associated with CME, may not necessarily be associated with decreased visual acuity. Symptomatic ERM, as in non-uveitic eyes, may be approached with standard PPV and membrane peeling techniques provided inflammation is well-controlled, with addition of periocular and/or intravitreal steroids intraoperatively, especially in the presence of CME. Final visual acuity may be limited by the development of cataract and membrane recurrence.30,31
Retinal Detachment
Uveitis was found to be an independent risk factor for rhegmatogenous retinal detachment with a prevalence of 3.1 percent in one large uveitic population.32 While a final reattachment rate of 88 percent was achieved using either scleral buckling or vitrectomy techniques, 67 percent had a final visual acuity worse than 20/200, attributable in part to the high incidence of preoperative proliferative vitreoretinopathy.
Complicated retinal detachments are frequently encountered in patients with necrotizing viral retinitis (CMV, ARN, PORN). These may be successfully repaired in a high percentage of cases with vitrectomy techniques, and frequently require long-term tamponade with silicone oil (See Figure 1).33.34 Tractional complications not infrequently seen in patients with ocular toxocariasis, toxoplasmosis, pars planitis and other entities associated with retinal neovascularization are best managed PPV and may be facilitated by bimanual membrane dissection (See Figure 2).35
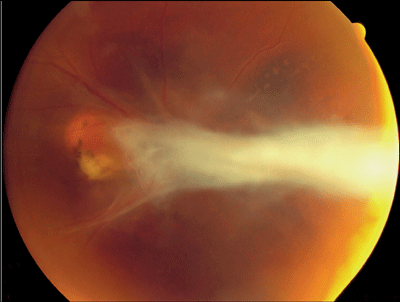 |
| Figure 2. Prominent epiretinal fibrosis and tractional retinal detachment in a patient with Toxocara canis. |
Hypotony
Selected cases of hypotony due to uveitis are amenable to surgical therapy, especially those in which irreversible damage to the ciliary processes has not occurred. Chronic uveitis can lead to the development of cyclitic membranes and hypotony, both by increasing outflow through ciliary traction and by decreasing secretion through direct ciliary epithelial damage. Should anti-inflammatory therapy fail to reverse hypotony, preoperative assessment of the ciliary body with UBM is helpful in identifying the presence or absence of the ciliary processes and the location and thickness of the epiciliary membranes. PPV with 360-degree scleral indentation, vitreous base dissection and bimanual epiciliary membranectomy may affect a sustained rise in intraocular pressure with improved vision in eyes with intact ciliary processes. In those with atrophic ciliary processes, silicone oil being useful in preventing phthisis.36
Sustained Drug Delivery
The Food and Drug Administration has approved, Retisert, (Bausch & Lomb), a 0.59-mg fluocinolone acetonide, non-biodegradable, intravitreal sustained drug-delivery implant for the treatment of chronic non-infectious posterior uveitis based on the findings of two pivotal studies.37,38 Device implantation is similar to that of the Vitrasert (ganciclovir, B&L) implant and may be more facile due to its relatively smaller size. Surgical complications are similar and include retinal detachment, intraocular infection, vitreous hemorrhage and wound dehiscence. As expected, continuous steroid exposure produced cataract requiring surgery in 90.3 percent of implanted eyes and elevated IOP in 64 percent, with approximately 32 percent of patients expected to require filtering surgery. With judicious patient selection, the benefits can be significant, with a reduction in inflammatory recurrence from the 40- to 54-percent range to the 7- to 14-percent range at 34 weeks. Visual acuity improved three lines or more among 19 to 21 percent of implanted patients after 34 weeks with 15 to 17 percent of these individuals maintaining this level of improvement after one year. Moreover, the requirement for systemic corticosteroid therapy was reduced from a 47 to 63 percent range to a 5 to 10 percent range at 34 weeks.
Vitreoretinal surgery is an essential tool in the management of chronic uveitis that presents a diagnostic dilemma, as it allows the differentiation among infectious, inflammatory and neoplastic etiologies, and so, specific directed treatment. Vitreoretinal techniques are useful in the management complicated cataract, vitreous opacification, endophthalmitis, lens-induced uveitis, and structural complications, such as retinal detachment and hypotony, and are possibly relevant to outcomes of improved vision, inflammatory control, and reduced CME.2
More convincing evidence for the role of PPV in the management of uveitis, especially in the light of expanding therapeutic indications with sustained drug delivery modalities, awaits more critical evaluation in well-designed, randomized, controlled, collaborative trials or hypothesis-based case series with defined outcome measures.
Dr. Vitale is chief of the Uveitis Service and a member of the Vitreoretinal Division at the John A. Moran Eye Center, University of Utah. Contact him at Albert.Vitale@hsc.utah.edu. He is a consultant to Bausch & Lomb.
1. Diamond JG, Kaplan HJ. Lensectomy and vitrectomy for complicated cataract secondary to uveitis. Arch Ophthalmol 1978;19:1798-1804.
2. Becker M, Davis JL. Vitrectomy in the treatment of uveitis. Am J Ophthalmol 2005;140:1096-1105.
3. Flynn HW Jr, Davis JL, Culbertson WW. Pars plana lensectomy and vitrectomy for complicated cataracts in juvenile rheumatoid arthritis. Ophthalmology 1988;95:1114-1119.
4. Androudi S, Ahmed M, Fiore T, et al. Combined pars plana vitrectomy and phacoemulsification to restore visual acuity in patients with chronic uveitis. J Cataract Refract Surg 2005;31:472-478.
5. Amino, K, Yamakawa R. Long-term results of out-of-the-bag intraocular lens implantation. J Cataract Refract Surg 2000;26:266-270.
6. Holland GN, Van Horn SD, Margolis TP. Cataract surgery with ciliary sulcus fixation of intraocular lenses in patients with uveitis. Am J Ophthalmol 1999 Jul;128:21-30.
7. Borgioli, M, Coster DJ, Fan RF, Henderson J, et al. Effect of heparin surface modification of polymethylmethacrylate intraocular lenses on signs of postoperative inflammation after extracapsular cataract extraction. One-year results of a double-masked multicenter study. Ophthalmology 1992;99:1248-1254; discussion 1254-1255.
8. Alió JL, Chipoint E, BenEzra D, Fakhry MA. Comparative performance of intraocular lenses in eyes with cataract and uveitis; the International Ocular Inflammation Society Study Group of Uveitic Cataract Surgery. J Cataract Refract Surg 2002;28:2096-2108.
9. Freyler H, Velikay M. Kitrektomie bei Uveitis. Klin Monatsbl Augenheilkd 1984;185:263-267.
10. Leuenberger PM. L'interet de la vitrectomie dans le traitment des uveitis posterieures. Klin Monatsbl Augenheilkd 1984:184:445-448.
11. Eckardt C, Bacskulin A. Vitrectomy in intermediate uveitis. Dev Ophthalmol 1992;23:232-238.
12. Heiligenhaus A, Bornfeld N, Foerster MH, Weissing A. Long-term results of pars plana vitrectomy in the management of complicated uveitis. Br J Ophthalmol 1994;78:549-554.
13. Verbraeken H. Therapeutic pars plana vitrectomy for chronic uveitis: A retrospective study of the long-term results. Graefes Arch Clin Exp Ophthalmol 1996; 234:288-293.
14. Scott RA, Sullivan PM, Aylward GW, Pavesio CE, et al. The effect of pars planitis complicated by vitreous hemorrhage: Visual outcome and long-term follow-up. Am J Ophthalmol 2001;131:514-515.
15. Sikic J, Suic JP. Surgical treatment of uveitis. Caoll Antropol 2001;25 Suppl: 71-76.
16. Soylu M, Demircan N, Pelit A. Pars plana vitrectomy in ocular Behçet's disease. Int Ophthalmol 2001;24:219-223.
17. Stavrou, P, Baltatzis S, Letko E, Samson CM, Christen W, Foster CS. Pars plana vitrectomy in patients with intermediate uveitis. Ocul Immunol Inflamm 2001;9:141-151.
18. Ieki Y, Kiryu J, Kita M, et al. Pars plana vitrectomy for vitreous opacity associated with ocular sarcoidosis resistant to medical treatment. Ocul Immunol Inflamm 2004;12:35-43.
19. Trittibach P, Koerner F, Sarra GM, Garweg JG. Vitrectomy for juvenile uveitis: Orognostic factors for the long-term functional outcome. Eye 2005 Apr 1;[Epub ahead of print]
20. Nolle B, Eckardt C. Vitrectomy in multifocal chorioretinitis. Ger J Ophthalmol 1993;2:14-19.
21. Bovey EH, Herbort CP. Vitrectomy in the management of uveitis. Ocul Immunol Inflamm 2000;8:285-291.
22. Kaplan HJ. Surgical treatment of intermedial uveitis. Dev Ophthalmol 1992;23:185-189.
23. Foster CS. Vitrectomy in the management of uveitis. Ophthalmology 1988;95:1011-1012.
24. Dugel PU, Rao NA, Ozler S, Liggett PE, et al. Pars plana vitrectomy for intraocular inflammation-related cystoid macular edema unresponsive to corticosteroids. A preliminary study. Ophthalmology 1992; 99:1535-1541.
25. Kiryu J, Kita M, Tanabe T, Yamashiro K, et al. Pars plana vitrectomy for cystoid macular edema secondary to sarcoid uveitis. Ophthalmology 2001;108:1140-1144.
26. Wiechens B, Nolle , Reichelt JA. Pars-plana vitrectomy in cystoid macular edema associated with intermediate uveitis. Graefes Arch Clin Exp Ophthalmol 2001;239:474-481.
27. Antcliff RJ, Spalton DJ, Stanford MR, Graham EM, et al. Intravitreal triamcinolone for uveitic cystoid macular edema: An optical coherence tomography study. Ophthalmology 2001;108:765-772.
28. Androudi S, Letko E, Meniconi M, Papadaki T, et. al. Safety and efficacy of intravitreal triamcinolone acetonide for uveitic macular edema. Ocular Immunol Inflamm 2005;13:205-212.
29. Kok H, Lau C, Maycock N, McCluskey P, et al. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology 2005;112:1916-1921.
30. Dev S, Mieler WF, Pulido JS, Mittra RA. Visual outcomes after pars plana vitrectomy for epiretinal membranes associated with pars planitis. Ophthalmology 1999;106:1086-1090.
31. Kiryu J, Kita M, Tanabe T, Yamashiro K, et al. Pars plana vitrectomy for epiretinal membrane associated with sarcoidosis. Jpn J Ophthalmol 2003;47:479-483.
32. Kerkhoff FT, Lamberts QJ, van den Biesen PR, Rothova A. Rhegmatogenous retinal detachment and uveitis. Ophthalmology 2003;110:427-431.
33. Blumenkranz M, Clarkson J, Culbertson W, Flynn HW, et al. Vitrectomy for retinal detachment associated with acute retinal necrosis. Am J Ophthalmol 1988;106:426-429.
34. Kreiger AE, Gonzales CR. Management of combined inflammatory and rhegmatogenous retinal detachments (AIDS and ARN). In: Ryan SJ, ed. Retina, vol 3, St. Louis: Moseby, 2005.
35. Amin HI, McDonald HR, Han DP, Jaffe GJ. Vitrectomy update for macular traction in ocular toxocariasis. Retina 2000;20:80-85.
36. Morse LS, McCuen BW. The use of silicone oil in uveitis and hypotony. Retina 1991;11:399-404.
37. de Smet MD, Gunning F, Feenstra R. The surgical management of chronic hypotony due to uveitis. Eye 2005;19:60-64.
38. Data on file. Bausch and Lomb, Inc.



