Ophthalmology is a constantly advancing surgical subspecialty. For example, over the past half century, cataract surgery techniques have evolved dramatically. Techniques have progressed from intracapsular lens removal, to extracapsular lens removal, to the modern day clear-cornea, sutureless, phacoemulsification cataract procedure. Operative times have decreased, postoperative recovery times have improved, and outstanding visual results have become the norm.
Retina surgery has had an equivalent advancement in techniques and instrumentation that have dramatically broadened the spectrum of diseases that can be treated with a vitrectomy. Simultaneously, operative times have decreased and postoperative visual outcomes have improved. In particular, recent instrumentation advancements have made a remarkable difference in the way vitrectomy surgery is performed. Flexibility in the selection of instrument size is significantly improving the operative process for surgeons and patients.
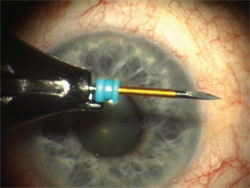
Figure 1. Trocar cannula on a beleveled blade to be used to enter the eye through the conjunctiva and sclera.
Instruments Push Surgical Evolution
Modern vitrectomy surgery has classically been performed with 20-ga. instrumentation. Twenty-ga. surgery has been advanced with the development of high-speed vitreous cutters, highly specified surgical instruments and the use of wide-angle viewing systems. These advancements are examples of ways that improvements in instrumentation have allowed retina surgeons to perform easier manipulations of the macula and the peripheral retina in repairing macular holes, macular puckers and tractional as well as rhegmatogenous retinal detachments.1
Initiating a 20-ga. vitrectomy procedure involves making nasal and temporal incisions in the conjunctiva, applying diathermy to control bleeding episcleral vessels, and then incising the globe with an MVR blade to make three scleral incisions through the pars plana to allow access to the vitreous cavity and the posterior segment of the eye. One sclerotomy is used for infusion, one for illumination and the other for the vitrector or other instrument. The infusion cannula needs to be temporarily sutured into place at one of the sclerotomy sites, and frequently the other sclerotomy sites need to be slightly enlarged during the case to allow for a slight variation in the diameter of some of the instruments used during the procedure. Once the infusion cannula is sutured into position, it is not moved throughout the case. Finally, at the conclusion of the case, the sclerotomies are individually closed with sutures and the conjunctiva is also placed back into position with dissolvable sutures.
In contrast, a 25-ga. procedure is initiated by placing trocars through the conjunctiva, sclera and pars plana to allow repeated access to the vitreous cavity. Three trocars are placed; one for the infusion, one for the light pipe and one for the vitrector or other surgical instrument.
There is flexibility in the placement of the 25-ga. infusion cannula and instruments. For example, the 25-ga. infusion cannula may be moved during surgery to another trocar site and instrumentation can be inserted through the traditional infero-temporal trocar to afford better access and approach with 25-ga. instrumentation; this is not possible with the fixed 20-ga. infusion cannula. There are no incisions in the conjunctiva, no cuts into the sclera that require suturing, and the infusion cannula does not require suturing to the sclera. At the conclusion of the case, the trocars are removed and the wounds self-seal without sutures. Overall, patients have an improved intraoperative and postoperative experience with 25-ga. surgery compared to 20-ga. surgery.
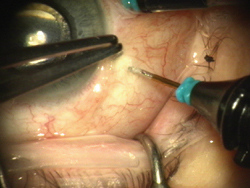 |
| Figure 2. The trocar is placed into the eye 3.5-mm posterior to the limbus. |
Several aspects of the 20-ga. procedure are significantly improved when 25-ga. surgery is performed. There is a decrease of approximately nine minutes in the operative time when performing 25-ga. vitrectomies due to decreased opening and closing times.2 In addition, there is no irregular postoperative astigmatism secondary to sutures and wound healing with the 25-ga. vitrectomy. This improves the early postoperative visual acuity for the patient. Further, 25-ga. surgery has less postoperative inflammation associated with the procedure. Therefore, treatment with anti-inflammatory medications for two to three weeks postoperatively, as is needed in 20-ga. surgery, is not as crucial. Finally, as sutures are not used with the 25-ga. technique, patients no longer complain of a foreign-body sensation as the conjunctival sutures dissolve during the first one to three weeks after 20-ga. vitrectomy surgery.
Increasing Indications
A variety of retinal conditions can be treated easily with 25-ga. vitrectomy surgery. The list includes, but is certainly not limited t macular holes; macular puckers; straightforward retinal detachments and vitreous hemorrhage from a variety of sources including diabetes, vascular occlusions, trauma, retinal detachment and macular degeneration. All of these conditions have been reported to be safely treated with 25-ga. vitreous surgery.3 In addition, long-term efficacy and safety have been reported with 25-ga. surgery.4 As surgeons gain experience with 25-ga. surgery, the list of potential 25-ga. cases continues to broaden.
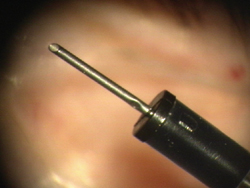
Figure 3. Infusion cannula.
Pros and Cons
There are, however, several aspects of 20-ga. surgery that still provide significant advantages over 25-ga. surgery in the most complex cases. The first advantage of 20-ga. surgery is that the light source is significantly brighter compared to a 25-ga. light pipe. Fiber-optic transmission limits the illumination that can be transmitted through a 25-ga. light pipe compared to a 20-ga. light pipe. Brighter light makes surgical manipulation of the vitreous and retina easier. In addition, in complex cases, lighted knives, lighted picks and lighted laser probes allow the surgeon to replace the typical light pipe, which allows for bimanual manipulations in the eye. This technique is not easily available to perform in 25-ga. surgery unless additional sclerotomies are made to allow for other sources of intraocular chandelier illumination.
Another advantage of 20-ga. surgery is the ability to maneuver the eye during the vitrectomy procedure. Complex vitreous surgery requires rotating the eye to the extreme positions during the vitrectomy to gain access to the far peripheral vitreous and retina. Twenty-ga. instruments are rigid and allow increased torque to be applied while manipulating the eye during surgery. Twenty-five ga. instruments are less rigid than 20-ga. instruments, and moving the eye becomes more difficult, limiting some of the maneuvers that can be performed during 20-ga. cases. The 25-ga. trocars lengthen the pivot point of instrumentation (as compared with 20-ga. vitrectomy), necessarily limiting the excursion of 25-ga. instruments within the vitreous cavity. Complex retinal detachments from giant retinal tears, proliferative vitreoretinopathy, advanced diabetic membranes and pediatric vitreous surgery are easier to perform using 20-ga. surgery compared to 25-ga. surgery, especially if the surgeon is less experienced with 25-ga. techniques. Another advantage to the 20-ga. surgery is that there is no postoperative hypotony from leaking sclerotomy sites, which can occur with 25 ga. cases.
Pearls
There are some surgical pearls, however, that enable surgeons to perform more complex retina surgery using 25-ga. surgery. The use of wide-angle viewing systems permits visualization of the peripheral vitreoretinal pathology with less need to torque the eye to extreme positions. In addition, switching the placement of the 25-ga. infusion cannula during surgery permits access of 25-ga. instrumentation to areas not easily reached when the infusion cannula is fixed, as in 20-ga. pars plana vitrectomy. Furthermore, hypotony can be reduced in 25-ga. cases by performing an air-fluid exchange at the end of the case. Leaving the eye filled with air seems to significantly reduce early postoperative pressure problems. Nevertheless, 20-ga. surgery still provides advantages over 25-ga. surgery for the most complex cases.
 |
| Figure 4. Infusion cannula locks into the trocar. |
New advances in 25-ga. instrumentation are developing rapidly. New composite materials are being utilized to develop stiffer instrumentation. Further, improving fiber-optic transmission technology permits thicker 25-ga. endoillumination casing by narrowing the fiber optic itself. Illumination advances such as xenon illuminators have the capacity to deliver a larger number of lumens safely even through more narrow 25-ga. fiber-optics. As newer, stiffer 25-ga. instruments are developed, the gap between 20-ga. and 25-ga. surgery techniques is narrowing.
Recently, 23-ga. vitrectomy has been developed and is slowly being utilized in retina surgery. Theoretically, 23-ga. surgery has many of the advantages of 25-ga. vitrectomy, including being sutureless, allowing for decreased opening and closing surgical times. In addition, the larger-diameter instruments are more rigid than 25-ga. instruments, allowing for easier torque of the eye and less unwanted bending of the instruments during the procedure. Further, the diameter of the light pipe enables brighter illumination than 25-ga., improving another weakness of 25-ga. surgery. The jury is still out on this new and emerging technology. Perhaps 23-ga. surgery will prove to be the perfect combination of the benefits of 20-ga. and 25-ga.
The standard of care in vitreoretinal surgery is evolving; recent advances have dramatically changed the approach to retina surgery. Technological advances and surgeon adaptation to the ever-changing techniques will continue to guide the revolution.
Dr. Kaiser is an assistant clinical professor at Wills Eye Hospital, Retina Service, and Thomas Jefferson University. Contact him at KaiserRick@ aol.com.
1. Spitznas M. A binocular indirect ophthalmomicroscope (BIOM) for non-contact wide-angle vitreous surgery. Graefes Arch Clin Exp Ophthalmol 1987;225:13-5.
2. Fujii GY, De Juan E Jr, Humayun MS, Pieramici DJ, Chang TS, Awh C, Ng E, Barnes A, Wu SL, Sommerville DN. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology 2002;109:1807-12.
3. Lakhanpal RR, Humayun MS, de Juan E Jr, Lim JI, Chong LP, Chang TS, Javaheri M, Fujii GY, Barnes AC, Alexandrou TJ. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology 2005;112:817-24.
4. Ibarra MS, Hermel M, Prenner JL, Hassan TS. Longer-term outcomes of transconjunctival sutureless 25-gauge vitrectomy. Am J Ophthalmol 2005;139:831-6.