Advances in retinal imaging modalities allow us to visualize posterior segment findings to a previously unmet degree. The clinical utility of these imaging techniques is constantly expanding, with new developments in machinery allowing for improvements in patient care. Better fields of view, image clarity/detail and modes of acquisition not only help during diagnosis of retinal disorders but also during screenings and follow-ups. Well-captured retinal images allow us to identify disease severity and stage, compare follow-up visit images to documented findings, track progression of disease between visits, and screen for pathology early. Additionally and importantly, the capture of clear retinal images allows for unambiguous communication between physicians and contributes to the education of trainees.
In this article we’ll explore a number of current retinal imaging modalities and their broadened utilities. It’s important to keep in mind that many of these imaging techniques and technologies are used in conjunction with each other to gain a fuller understanding of the extent of pathology in a given patient.
Fundus Photography
 |
This old standby has gone through several iterations in recent years that have increased its versatility and usefulness.
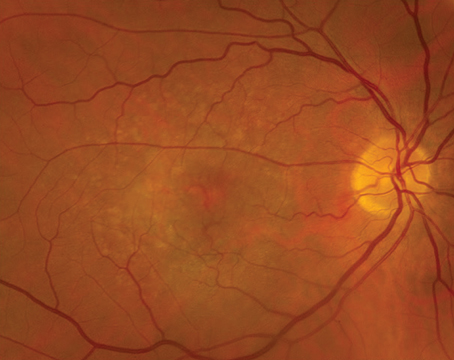
• Color. Standard color fundus photography continues to allow for the documentation of posterior pole findings including the macula and optic disc. These 20 to 50 degree images can be acquired rapidly and provide painless, reproducible, and high-resolution views that are true to size and color. However, these images are limited in their scope and are largely falling by the wayside in favor of widefield fundus imaging that allows for visualization of the retinal periphery, not just the macula and optic disc. Thirty-degree images just barely capture both the optic disc and macula in one image. Standard color fundus images can be useful if there’s a need to capture high-magnification images of the macula in the clinic or to get a true-to-scale, color image of the optic nerve.
 |
• Widefield. With the invention of widefield and ultra-widefield fundus imaging systems such as the non-contact tabletop Zeiss Clarus (Carl Zeiss Meditec AG; Jena, Germany) and Optos Daytona (Optos PLC; Dunfermline, United Kingdom), it’s become possible to quickly gather high-resolution information beyond the posterior pole. As the peripheral retina is the site of pathology in many retinal diseases, widefield fundus photography has proved transformational for early screening, diagnosis and monitoring of an expanded list of retinal diseases. Ultra-widefield imaging allows for imaging of 80 percent or more of the retinal surface area through a 200-degree retinal view. It’s now not only possible to assess more peripheral findings in diseases like diabetic retinopathy and macular degeneration, but these systems also allow for imaging and evaluation of choroidal masses, retinal vasculitis, choroidal dystrophies, hereditary retinal disorders and retinal vein occlusions outside the normal limited window of capture in standard fundus photography. Capture and documentation of peripheral lesions such as lattice degeneration, atrophic holes and retinoschisis allows for a more thorough and comprehensive assessment of extent of disease. Widefield fundus imaging has also allowed us to document previously unseen peripheral vascular pathology, thus widening insight into many adult and pediatric retinal diseases.1
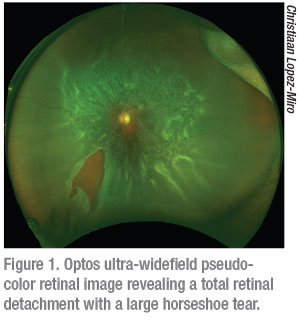
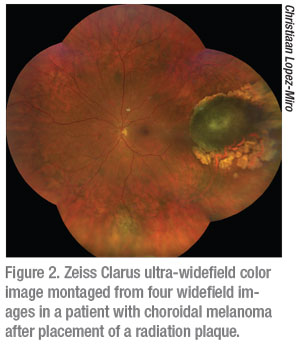
It is important to note that it’s still not possible to capture images from ora to ora and it is thus still possible to miss peripheral retinal pathology. When considering the difference between the Zeiss Clarus and Optos Daytona it helps to understand that the Optos is a scanning laser ophthalmoscope and therefore provides a pseudo-color representation of the fundus (Figure 1). The Zeiss Clarus, which automatically takes and montages two pictures to approximate the same field of view as Optos, is true-color (Figure 2). As such, the Clarus is especially useful in the evaluation of ocular tumors.
 |
It should also be noted that in patients with undilated eyes the Optos is still able to capture full images with relative ease. For uveitic patients in which the iris has scarred down,
Optos is still able to capture a full field of view, allowing for proper assessment of the periphery. In pediatric patients it’s important to keep in mind that whereas the Clarus has a very bright flash that some children dislike, the SLO Optos doesn’t and, as a result, may be better tolerated by pediatric patients.
• Handheld. The advent of portable handheld fundus photography devices has made it possible to not only image newborns in the neonatal intensive care unit and infants in our clinic, but also to image bedridden adults and patients in the OR during exams under anesthesia. The RetCam 3 (Natus Medical Incorporated; Pleasanton, California), Phoenix Icon (Phoenix Technology Group; Pleasanton, California), and PanoCam (Visunex Medical Systems; Fremont, California) are widefield handheld fundus photography systems that allow for evaluation and documentation of retinal findings and changes through the periphery.2 The use of these devices has extended care significantly to newborns and infants with retinopathy of prematurity within the hospital. They also provide a unique opportunity for evaluation via telemedicine; stitched montages of images can be used to help in this effort.
 |
All three of these devices are contact systems and require the application of gel onto the cornea. Issues with stability can affect image acquisition. Whereas the RetCam 3 and Phoenix Icon are capable of fluorescein angiography, the PanoCam is not. Unlike the RetCam 3 and the Phoenix Icon, the PanoCam is wireless and is thus easier to maneuver.
Fluorescein Angiography
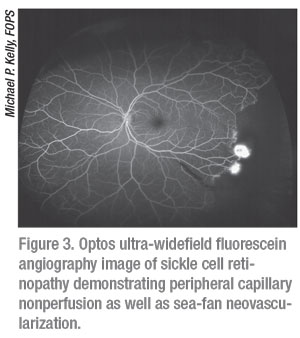
Through the use of either IV or oral fluorescein, fluorescein angiography allows for the visualization and documentation of flow through the retinal vasculature over time. FA is able to reveal the presence of aberrant vasculature all the way through the periphery (Figure 3). Neovascularization, areas of non-perfusion, abnormal vascular findings and areas of leakage can all be detected via FA. There are both tabletop as well as portable options for obtaining FA images. Widefield devices such as the Optos California FA, RetCam 3 and Phoenix Icon are all capable of FA. Heidelberg Spectralis (Heidelberg Engineering; Heidelberg, Germany) is also capable of FA, and has a widefield lens that can be used with the machine. It should be noted that FA images can be obscured by dye leakage; for example, in cases of choroidal neovascularization the fluorescein can leak too much to be able to properly visualize the structure.3 It’s also important to keep in mind that with oral FA, which is generally only used in children who can’t tolerate IV FA or for whom it’s too difficult to find a vein, it’s impossible to obtain arterial phase images with oral FA.4 Because we can only obtain late-phase angiographic images with oral FA, it is generally only useful in the evaluation of conditions in which there is late leakage such as instances of neovascularization or cystoid macular edema (which can reveal accumulation of dye leakage in the cystic spaces of the macula).
Fundus Autofluorescence
 |
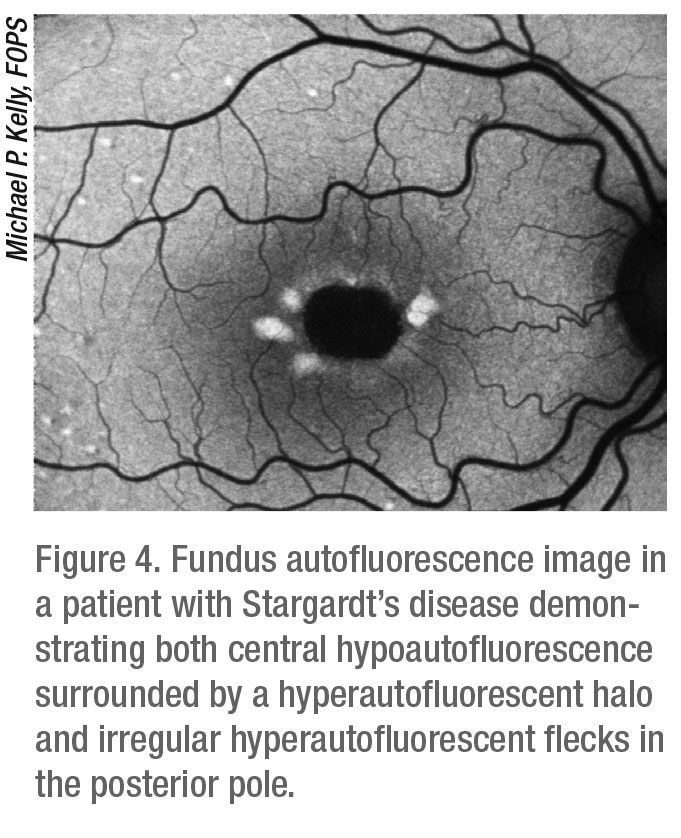
Fundus autofluorescence takes advantage of the fluorescent properties of lipofuscin under certain wavelengths of light to offer views of autofluorescence patterns that reveal the health of the retinal pigment epithelium. In certain retinal disease states, such as age-related macular degeneration, macular dystrophies and retinitis pigmentosa, there is abnormal lipofuscin buildup in unhealthy RPE cells which are unable to properly phagocytose these accumulating granules. In healthy retinas with normal distribution and turnover of lipofuscin in the RPE cell layer, the FAF appears as a black-and-white image of the fundus. Areas in which unhealthy RPE cells can’t properly metabolize accumulating lipofuscin will appear brighter. Areas in which RPE cells have died will appear black. FAF is a useful diagnostic tool often used as a complement to FA to diagnose, evaluate and monitor progression of diseases such as AMD, hereditary retinal dystrophies, optic nerve head drusen and astrocytic hamartoma.5
Devices that can perform FA can also perform FAF as they use the same wavelength of light. Widefield systems such as the Optos California FA and the Zeiss Clarus can perform FAF, with widefield FAF proving to be particularly useful for the evaluation of certain intraocular tumors and retinal dystrophies. The Heidelberg Spectralis is also capable of FAF with the widefield lens as well as the standard 30-degree and 55-degree lenses (Figure 4). Many standard 50-degree color fundus cameras such as Nikon, Topcon and Canon can also perform both FA and FAF.
Indocyanine Green Angiography
 |
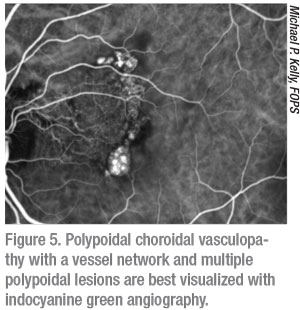
Using a different wavelength of light than both FA and FAF, indocyanine green angiography allows us to see past the retina and into the choriocapillaris and choroid. ICG dye is injected intravenously, flows through the retinal and choroidal circulation, and is then imaged with infrared light that penetrates the retinal layers as the choroidal vasculature fluoresces. As such, ICG provides an angiogram of the choroid and is most useful for evaluation of diseases such as polypoidal choroidal vasculopathy (Figure 5), choroidal neovascularization, intraocular tumors, retinal angiomatous proliferation and central serous chorioretinopathy. While some widefield cameras, such as Optos California ICG, can do ultra-widefield ICG, some retinal physicians find it useful to obtain magnified ICG images targeted to the area of interest. On the other hand, widefield ICG can prove to be very useful in the evaluation of vascular alterations in certain uveitic conditions, especially chorioretinal inflammatory disorders such as multifocal choroiditis and birdshot retinochoroidopathy.
 |
It’s possible to obtain both static ICG images (useful for the evaluation of intraocular tumors and uveitis) as well as dynamic ICG videos (useful for evaluation of NAMD and choroidal neovascularization). In presentations of NAMD in which there are feeder vessels, polypoidal choroidal vasculopathy, or retinal angiomatous proliferation dynamic information regarding flow can be particularly helpful.
Optical Coherence Tomography
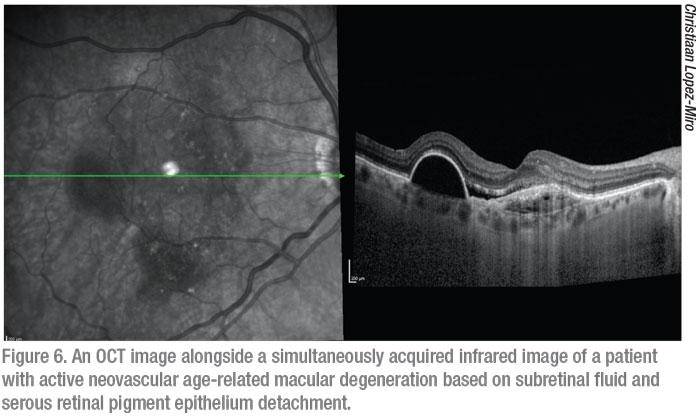
Since its introduction more than two decades ago, posterior segment OCT has become a mainstay in virtually all workups in the retina clinic. Through the use of near infrared wavelength light and the principle of low coherence interferometry, OCT is able to discern the different layers of the retina to provide detailed, high-resolution, 2-D cross-sectional images through the volume of the retina. Morphological changes, as well as alteration, deformation and loss of structure are made clear via OCT. These non-invasive, non-contact scans inform diagnosis, allow for monitoring of disease progression and let us evaluate the response to treatment. The most commonly used type of OCT currently is spectral-domain OCT, which uses a short wavelength of light and a spectrometer as the detector. There are a number of different SD-OCT devices on the market with differing axial resolutions, scanning speeds and features.6 Some of the most widely used systems are the Zeiss Cirrus, the Heidelberg Spectralis and the OptoVue Avanti (Opto-Vue; Fremont, California). Swept-source OCT uses a laser that sweeps over a broad range of longer wavelengths allowing for greater penetration through the retina for evaluation of the choroid. SS-OCT also measures the interference spectrum with photodetectors instead of a spectrometer, allowing for faster scanning speeds and the ability to obtain wider field B scans. The Topcon DRI OCT Triton (Topcon, Oakland, N.J.) and the Zeiss Plex Elite 9000 are two SS-OCT systems but are found much less commonly in clinics than SD-OCT systems, which have seemed to retain favor. At this time the Zeiss Plex Elite 9000 is only marketed for research use. Alongside OCT images, systems capture an infrared fundus image via a secondary light source that corresponds with the volume through which the OCT scans were obtained (Figure 6).
 |
With the development of the handheld Bioptigen/Leica Envisu C2300 OCT system (Leica Microsystems, Durham, North Carolina) it has become possible to image newborns in the NICU and infants in clinic. This is especially useful for diseases such as ROP, X-linked retinoschisis, Coats’, non-accidental trauma and familial exudative vitreoretinopathy. The Bioptigen head is lightweight and well-balanced which makes it easy to maneuver. While the handheld system provides a good virtual biopsy of the retina it doesn’t allow for macular thickness maps or RNFL measurements. It should be noted that it’s possible to adjust the reference arm to reduce clipping, manually adjust the focus, and adjust the scan length and density when using the handheld Bioptigen and tabletop OptoVue AngioVue. This is especially useful when imaging pediatric patients in which the axial length, refractive error, astigmatism and corneal curvature change with age.7
Recently, Leica launched EnFocus, an intraoperative OCT system (See Product News, p. 62 for more information). There’s also one OCT device that’s in the FDA approval process, the Heidelberg FLEX, in which the camera head is attached to a maneuverable armature allowing for OCT imaging in the OR (Figure 7).
 |
Optical Coherence Tomography Angiography
 |
In recent years the development of OCTA has allowed us to obtain three-dimensional, depth-encoded images that can clearly reveal the vessels in which there is flow in the different vascular layers of the retina and choroid. OCTA is an extension of traditional OCT that takes multiple B-scans through each point along the volume of the retina; the plotting of the signal difference from the moving erythrocytes between B scans taken in the same location allows us to obtain a map of blood flow through the retina. In this way it’s possible to assess for perfusion defects, abnormal vessel distribution, and pathologic vascular layer features. Unlike FA, OCTA doesn’t require either the use of IV or oral fluorescein. OCTA images provide more detail than FA images and allow for assessment of flow and vasculature at all levels of the retina and choroid. It should be noted however that OCTA images cannot detect real time leakage from damaged vessels and have a smaller field of capture (either 3 x 3-mm, 6 x 6-mm, or 12 x 12-mm scan area) thus limiting peripheral and widefield visualization.
OCTA allows for both qualitative and quantitative evaluation of depth-encoded retinal vasculature. Not only is it useful for visualizing and identifying aberrant vasculature such as neovascular membranes, but OCTA can quantify and compare vessel density and FAZ area to better understand the extent of pathology at baseline as well as monitor disease progression.8
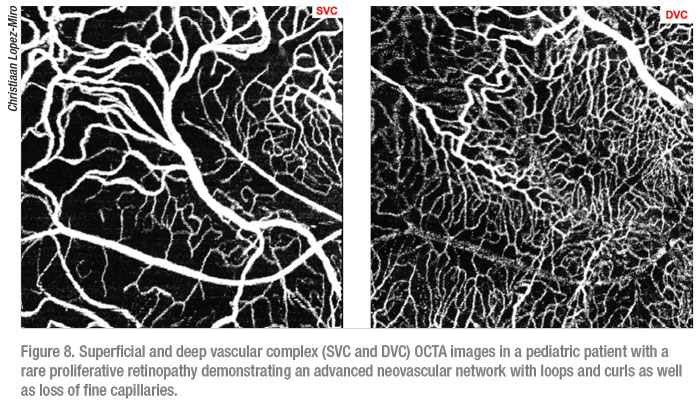 |
The four tabletop OCTA devices on the market all differ in scan speed and resolution: the OptoVue Angio-Vue; Zeiss Cirrus 6000 with Angioplex; Zeiss Cirrus 5000 with AngioPlex; and Heidelberg Spectralis. Keep in mind that all three devices define their superficial, deep and avascular layers differently. The Heidelberg Spectralis FLEX, which is currently seeking FDA approval, has made it possible to obtain OCTA images in the OR during exams under anesthesia. This has proven to be particularly useful in pediatric patients with retinal diseases with known neovascular components such as ROP, Coats’, and FEVR who often have trouble fixating long enough to obtain a clear OCTA image (Figure 8). It should also be noted that while dilation isn’t necessary to obtain an OCTA image in adults it makes an appreciable difference in the ability to capture a clear scan in children.
In conclusion, advancements in these retinal imaging modalities continue to expand our ability to diagnose, monitor, and treat retinal diseases in our patients. Through the use of these modalities, often used in conjunction in some combination, we are able to screen for and detect diseases earlier than ever and quickly identify progression in disease that might require changes to treatment plans. The development of new instrumentation and imaging techniques continues to have tremendous impact on access to care, our understanding of retinal disease and clinical practice. REVIEW
Ms. Ponugoti is a medical student completing two research years in the ophthalmology department at Duke University Medical School under the guidance of Dr. Vajzovic, who is an associate professor of ophthalmology at Duke. Mr. Kelly is the director of Duke Eye Imaging.
Dr. Vajzovic receives research funding from Heidelberg. The other authors have no financial interest in the products discussed.
1. Nagiel A, Lalane RA, Sadda SR, Schwartz SD. Ultra-widefield fundus imaging: A review of clinical applications and future trends. Retina 2016;36:4:660-78.
2. Callaway NF, Mruthyunjaya P. Widefield imaging of retinal and choroidal tumors. International Journal of Retina and Vitreous 1998;5(Suppl 1):1-10.
3. Mastropasqua R, Di Antonio L, Di Staso S, et al. Optical coherence tomography angiography in retinal vascular diseases and choroidal neovascularization. J Ophthalmol 2015;2015:343515.
4. Hara T, Inami M, Hara T. Efficacy and safety of fluorescein angiography with orally administered sodium fluorescein. Am J Ophthalmol 1998;126:4:560–564.
5. Oishi A, Miyata M, Numa S, et al. Wide-field fundus autofluorescence imaging in patients with hereditary retinal degeneration: A literature review. Int J Retin Vitr 2019;5:23.
6. Popescu DP, Choo-Smith LP, Flueraru C, et al. Optical coherence tomography: Fundamental principles, instrumental designs and biomedical applications. Biophys Rev 2011;3:3:155.
7. Cook A, White S, Batterbury M, Clark D. Ocular growth and refractive error development in premature infants with or without retinopathy of prematurity. Invest Ophthalmol Vis Sci 2008;49:12:5199-207.
8. Kashani AH, Chen C-L, Gahm JK, et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog Retin Eye Res 2017;60:66-100.