Anti-vascular endothelial growth factor therapy for wet age-related macular degeneration has been incredibly effective, as demonstrated both by large-scale clinical trials1-4 and our real-world experience, with about 94 to 95 percent of patients losing fewer than 15 ETDRS letters after a year.1 Our therapy for wet AMD may be a victim of its own success, however: The first-line anti-VEGF injections are so successful, there hasn’t been a pressing need to perform large, randomized studies on how to properly treat the percentage of patients who fail their first anti-VEGF therapy. This leaves retinal specialists and comprehensive ophthalmologists with the task of developing their own protocols for second-line therapy via experience. In this article, I’ll describe how I approach wet AMD patients who don’t succeed with their initial therapy, and also review some of the pertinent literature.
Defining Suboptimal Response
To understand my second-line protocol, it helps to understand my initial treatment approach and how I define suboptimal responders.
My first anti-VEGF agent, used in almost all of my cases, is Avastin (bevacizumab). I start wet AMD cases on Avastin for a few reasons: it tends to work well; it’s inexpensive; it doesn’t require preauthorization from the patient’s insurance company; and my institution does not participate in the Lucentis (ranibizumab) and Eylea (aflibercept) sampling programs that allow on-label treatment at presentation. At the outset, I usually tell patients that I expect to see a steady improvement over time with their Avastin treatment. My clinical team will start the precertification process for Lucentis and Eylea during the first visit, so we know what our options are moving forward.
When the patient initially presents and is diagnosed with wet AMD, I always check an optical coherence tomography (OCT) scan and often get a baseline fluorescein angiogram. (I only have OCTA in one of my offices, but I suspect this technology will eventually replace fluorescein angiography for wet AMD.)
For the initial treatment, I use a treat-and-extend protocol in which I give the patient at least three to four monthly injections, without extending the interval, with the expectation that we’ll see consistent improvement anatomically and, hopefully, visually. I continue to treat at four-week intervals until the retina is dry, and then I extend by two-week intervals. I use OCT to follow the response to anti-VEGF treatment.
I monitor the patient’s treatment response very carefully using registered OCT scans. If I see persistent fluid on OCT, particularly if it appears to be impacting the vision, I’ll consider the patient a suboptimal responder to the drug. I expect to see consistent anatomic improvement; if I don’t, even if it’s only been one injection and the eye is noticeably worse or hasn’t improved, I’ll consider the response suboptimal.
The patient’s visual acuity and symptoms play a significant role in my decision making. If the patient has a shallow sliver of subretinal fluid and is still 20/20 without symptoms, he may be a suboptimal responder, but I often do not feel compelled to change my treatment plan.
Occasionally, a patient is treated for some time with a particular drug, but eventually he or she will either begin to have a diminished response (tachyphylaxis) or the disease will get more aggressive—it’s difficult to tell which of these two mechanisms is contributing to this effect. When this occurs, I consider the response suboptimal (though it may have been optimal previously) and I’ll alter treatment.
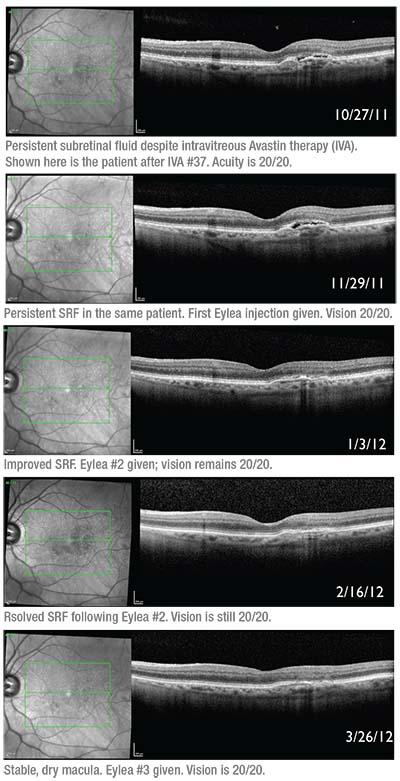 |
Fallback Plans
If my first-line treatment fails and I need to move on to something else, there are several options I consider. I will start by first switching the drug. If I don’t achieve the desired response, I’ll consider a higher dose of antiVEGF, such as a double-dose of Eylea or Lucentis. Failing this, I sometimes consider anti-VEGF treatment every two weeks instead of every four. Photodynamic therapy and, rarely, laser photocoagulation for extramacular choroidal neovascularization may also play roles.
• Switching drugs. Fortunately, we have three commonly used anti-VEGF drugs today—Avastin, Lucentis, and Eylea—with a fourth drug, brolucizumab, expected to hit the market in 2019. If a patient isn’t responding to a particular drug, even after only one injection, I will switch to one of the other drugs in an effort to achieve a better response. Pharmacologically, Lucentis has a greater binding affinity for VEGF than Avastin, and Eylea has a greater binding affinity than Lucentis. It follows that these three drugs may result in varied responses among the most recalcitrant cases of wet AMD.
In our practice, we reported an enhanced response in refractory wet AMD cases switched to Eylea. We performed a retrospective study of 353 eyes with wet AMD who had persistent fluid despite being treated with Lucentis 0.5 mg or Avastin 1.25 mg and were subsequently switched to Eylea 2 mg and followed for six months. Of the 353 eyes, 28 eyes in 28 patients had persistent fluid after an average of 20 regular injections of Lucentis or Avastin (range: 7 to 37 injections). At one month after the first Eylea injection, 89 percent (25 eyes) showed anatomic improvement and 18 percent (five eyes) were dry. Central subfoveal thickness improved from 295 to 272 μm (p<0.001). After an average of 4.4 Eylea injections (range: 3 to 6) over a period of six months, the central subfoveal thickness remained improved, 64 percent (18 eyes) showed anatomic improvement and a quarter of eyes (seven eyes) were dry. Visual acuity, however, didn’t improve in most patients at one month (20/69, p=0.64) or six months (20/76, p=0.49).5
A prospective, open-label, single-arm clinical trial from Retina Consultants of Houston, funded by Regeneron, demonstrated a beneficial effect from switching to Eylea. Forty-six patients who were incomplete responders to multiple Lucentis injections were switched. They were seen monthly and given 2 mg Eylea at months one, two and four. Pro re nata treatment was administered at months three and five if there was evidence of disease on SD-OCT.
Before the Eylea treatment, the patients had a mean vision of 74 letters (20/32), and an average central subfield thickness of 347 μm. Their ETDRS letter score remained stable throughout the trial, and at month six the mean change in best-corrected vision was +0.2 letters (range: -10 to +13, p=0.71). The mean CST decreased significantly at each visit, including -23.6 μm at the first month and -27.3 μm at month six. Seventy-one of 90 (79 percent) possible PRN injections were ultimately given, and a mean of 5.6 Eylea injections out of a maximum possible number of six were administered to each patient. Ten of the patients (22 percent) had no fluid on OCT at six months, and no patient lost more than 15 letters.6
Though some patients may respond to Eylea after incomplete response to either of the other two drugs, recent reports of inflammation after the use of Eylea give us pause. In a retrospective study we reported in 2014, we evaluated 20 cases of noninfectious inflammation after Eylea injections. In our report, the overall rate of inflammation was low, 20 out of 5,356 injections (0.37 percent), and 19 out of 844 patients (2.25 percent). All patients presented with decreased vision; three (15 percent) had pain; and two (10 percent) had conjunctival injection.7 After topical steroids, all but one patient regained their pre-injection visual acuity. Four patients resumed Eylea injections, and one of them developed inflammation again after five injections. There was also a more recent nationwide cluster of inflammation cases after Eylea in late 2017 and early 2018. Regeneron attributed this outbreak to the syringes packaged with the drug.8 The company is no longer distributing kits with those particular syringes.
Switching to Lucentis is also an option, as it’s an excellent drug with many clinical trials behind it that show good safety data. It also has the benefit of a prefilled syringe, and we recently presented an abstract at the annual meeting of the American Society of Retina Specialists that shows a lower rate of endophthalmitis with the Lucentis pre-filled syringe compared to the conventional Lucentis vial. This finding may be due to the reduced manipulation of withdrawing the drug from the vial.
In a retrospective study from Israel, physicians analyzed 114 eyes of 110 wet AMD patients who were switched from Avastin to Lucentis. Overall, the researchers say that switching didn’t achieve a significant change in visual acuity, and that even though there was a significant reduction in central retinal thickness after the first three injections, it wasn’t maintained by the end of the follow-up period. In 47.3 percent of the eyes, though, the mean central retinal thickness was reduced by at least 10 percent after the first three Lucentis injections and the reduction was maintained with additional injections. They note that eyes that lost at least 0.1 logMAR of visual acuity
before the switch were more likely to improve (p=0.013) and eyes with at least a 10-percent increase in central retinal thickness before the switch were more likely to improve anatomically (p=0.0003).9
• Increasing the dose. Physicians have also tried “super dosing” the antiVEGF drugs for recalcitrant AMD cases. This is my preferred strategy when I don’t achieve an adequate response from switching drugs. With Avastin, however, since it’s compounded in individual syringes, it’s difficult to get much more than 60 to 70 μl, which is only 10 to 20 μl more than the standard compounded dose. Thus, when I use a higher-dose drug, it is typically Lucentis 0.75 mg or 1 mg, or Eylea 3mgor4mg.
In one retrospective, interventional case study, researchers first switched AMD patients unresponsive to monthly Lucentis or Avastin to Eylea 2 mg every eight weeks. If there was resistance to that, patients were escalated to every four weeks. If there still was no response, the dose was increased to 4 mg every four weeks. Thirty-three eyes of 28 patients were ultimately treated with 4-mg Eylea every four weeks, and followed for a mean of 16 months. Subjects had a dry retina (no intraretinal or subretinal fluid) after a mean of 3.8 months of Eylea 4 mg every four weeks. Central foveal thickness, maximum foveal thickness, intraretinal fluid, subretinal fluid and retinal pigment detachment height decreased significantly one month after initiating the 4-mg Eylea, and the researchers reported the morphologic therapeutic effect was sustained until the last visit. Forty-five percent of eyes gained at least a line of vision. New geographic atrophy developed in 9 percent of eyes during follow-up, but there were no ocular or systemic adverse events.10
Rather than switching to high-dose Eylea, one group instead treated recalcitrant cases with a higher dose (2 mg) of Lucentis in a prospective fashion. The researchers split 88 patients into two groups: One group received the increased dose as-needed every four weeks (A), and another received it as-needed every six weeks (B). Seventynine patients completed the 12-month endpoint; they were given a mean of 11.6 (cohort A) and 8.6 (cohort B) treatments. Mean visual acuity gain over baseline was +2.5 letters at day seven (n=82), +3.7 letters at month one (n=87), +3.9 letters at month two (n=87) and +3.3 letters at month three (20/36 Snellen; p=0.001; n=86). The average BCVA gains of 4.1 letters following three monthly doses were sustained for a year in both cohorts. Anatomic improvements were sustained for 12 months for cohort A, but not for B, which demonstrated a gradual increase in mean central retinal thickness (p=0.03).11,12
Interestingly, the HARBOR study found that outcomes with Lucentis 2 mg were similar to those with Lucentis 0.5 mg at two years in treatment-naïve patients.13
• Modifying the dosing schedule. If someone fails all three medications, I’ll sometimes adjust the dosing schedule and treat him or her every two weeks instead of every four, in an attempt to achieve a better response. Due to insurance constraints, I’ll typically alternate injections between Avastin and one of the other on-label drugs.
A retrospective study of 18 eyes with refractory wet AMD after a mean of 22 intravitreal anti-VEGF injections found improvement in visual acuity after four bi-weekly alternating Lucentis/Avastin intravitreal injections (20/95 to 20/65 [p<0.001]). The mean central foveal thickness also improved (455 μm to 387μm [p=0.029]).14
• Photodynamic therapy. PDT can occasionally be a helpful adjunctive treatment modality to anti-VEGF injections for recalcitrant cases, or possibly in particular sub-classes of AMD. In our practice, if patients are still losing vision and demonstrating persistent exudation despite very aggressive anti-VEGF treatment, we’ll consider adding PDT.
In one paper, the Lucentis and PDT on polypoidal choroidal vasculopathy (LAPTOP) study, researchers randomized 93 patients with treatment-naïve polypoidal choroidal vasculopathy to receive either PDT or Lucentis monotherapy (0.5 mg) (three monthly injections) in a 1:1 ratio.15 Although the visual outcomes were better with Lucentis compared with PDT (p=0.004; month 24), PDT was able to induce regression of the PCV lesions.16
In another study, EVEREST, a Phase IV randomized, controlled trial that was the first to be primarily guided by indocyanine green angiography, researchers randomized 61 Asian patients to verteporfin PDT, Lucentis 0.5 mg or a combination of the two. Patients were administered verteporfin PDT/placebo and initiated with three consecutive monthly Lucentis/sham injections starting on day one, and were then re-treated (months three through five) based on predefined criteria. In the study, treatment with PDT plus Lucentis was superior to Lucentis alone for achieving regression of polyps at month six.17
• Laser photocoagulation. Anti-VEGF therapy is the standard of care for wet macular degeneration, but thermal laser occasionally plays a role for extramacular or peripapillary choroidal neovascularization. I will almost always start these patients on anti-VEGF therapy, to which they usually respond well. For cases that continue to progress, however, laser can be a useful adjunct. Of course, it’s critical to avoid the fovea and perifoveal area to prevent a symptomatic scotoma (and also possible future choroidal neovascularization). When photocoagulation is an option—and when it works—it can help decrease the injection burden for patients, and potentially decrease the frequency of visits.
• In the pipeline: brolucizumab and Cosopt. Brolucizumab is a new anti-VEGF drug targeting VEGF A that’s currently in trials. Its maker, Novartis, hopes that the drug may be able to demonstrate efficacy comparable to q8w Eylea but with q12w dosing. In the studies HAWK and HARRIER, just over half the brolucizumab patients were able to use that dosing regimen and still experience good efficacy.18 (For a complete discussion of brolucizumab and other agents in the pipeline, see this month’s article, “The nAMD Pipeline: Full but Not Fast,” on p. 38).
For wet-AMD eyes that have persistent exudation despite fixed-interval anti-VEGF injections, a pilot study from Wills Eye Hospital suggested that a combination of topical dorzolamide-timolol (Cosopt, Merck) and anti-VEGF injections might yield a beneficial effect. In the study, 10 eyes of 10 patients received a regimen of Cosopt b.i.d. and the same intravitreal injection of anti-VEGF, on the same schedule, that they were receiving before the study. Eight eyes received Eylea and two received Lucentis. The mean CST decreased from 419.7 μm at enrollment to 334.1 μm at the final visit (p=0.01). The mean maximum subretinal fluid height decreased from 126.6 μm at baseline to 49.5 μm at the final visit (p=0.02), and the mean maximum pigment epithelial detachment height decreased from 277.4 μm at enrollment to 239.9 μm (p=0.12). The mean logMAR visual acuity was 0.54 (a little worse than 20/63) at enrollment and 0.48 (slightly better than 20/63) at the final visit (p=0.60).19
Because of the success of this pilot study, several other researchers and I are conducting a larger-scale study using the same drug. In the study, subjects are randomly assigned to receive Cosopt or placebo along with their normally scheduled anti-VEGF injections at regular intervals as was done prior to enrollment. We’re currently enrolling patients in the study.
Though the mechanism behind Cosopt’s possible positive effect is unknown, it may have to do with how intravitreal anti-VEGF drugs are cleared from the eye. Some studies have suggested that outflow through the anterior chamber may play a role. The hypothesis is that, by decreasing aqueous production with Cosopt, outflow may also be reduced, possibly slowing the rate at which the anti-VEGF is cleared. This may allow the latter drug a longer working time.20
In conclusion, though anti-VEGF drugs have revolutionized the treatment of wet AMD, like driving on a straight road, we must often make adjustments along the way. There is minimal level-one evidence to guide us when treating suboptimal responders to anti-VEGF monotherapy. I hope this review of current strategies helps you face these challenging cases with greater confidence. REVIEW
Dr. Shah practices at Ophthalmic Consultants of Boston. He is an assistant professor of ophthalmology at Tufts University School of Medicine in Boston, a lecturer at Harvard Medical School, and the co-director of the Tufts/OCB Vitreoretinal Surgery Fellowship Program.
Dr. Shah is a sub-investigator in clinical trials sponsored by Regeneron and Genentech, and consults for Regeneron.
1. The CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897-1908.
2. Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
3. Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
4. Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
5. Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013 Aug;97:8:1032-5.
6. Wykoff CC, Brown DM, Maldonado ME. Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial). Br J Ophthalmol 2014;98:7:951-5.
7. Goldberg RA, Shah CP, Wiegand TW, Heier JS. Noninfectious inflammation after intravitreal injection of aflibercept: clinical characteristics and visual outcomes. Am J Ophthalmol 2014;158:4:733-737.
8. https://hcp.eylea.us/media/ioi.pdf. Accessed 10 July 2018.
9. Moisseiev E1, Katz G, Moisseiev J, et al. Switching treatment for neovascular age-related macular degeneration from bevacizumab to ranibizumab: Who is likely to benefit from the switch? Retina 2015;35:7:1323-30.
10. You QS, Gaber R, Meshi A, et al. High-dose high-frequency aflibercept for recalcitrant neovascular agerelated macular degeneration. Retina n2018;38:6:1156-1165.
11. Wykoff CC, Brown DM, Chen E. SAVE (Super-dose anti-VEGF) trial: 2.0 mg ranibizumab for recalcitrant neovascular age-related macular degeneration: 1-year results. Ophthalmic Surg Lasers Imaging Retina 2013;44:2:121-6.
12. Brown DM, Chen E, Mariani A, Major JC; SAVE Study Group. Super-dose anti-VEGF (SAVE) trial: 2.0 mg intravitreal ranibizumab for recalcitrant neovascular macular degeneration-primary end point. Ophthalmology 2013;120:2:349-54.
13. Ho AC, Busbee BG, Regillo CD, Wieland MR, Van Everen SA, Li Z, Rubio RG, Lai P; HARBOR Study Group. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014;121:11:2181-92.
14. Witkin AJ, Rayess N, Garg SJ, Maguire JI, Storey P, Kaiser RS, Hsu J, Vander JF, Ho AC. Alternating bi-weekly intravitreal ranibizumab and bevacizumab for refractory neovascular age-related macular degeneration with pigment epithelial detachment. Semin Ophthalmol 2017;32:3:309-315.
15. Oishi A, Kojima H, Mandai M, Honda S, Matsuoka T, Oh H, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol. 2013;156:644–51.
16. Oishi A, Miyamoto N, Mandai M, Honda S, Matsuoka T, Oh H, et al. LAPTOP study: A 24-month trial of verteporfin versus ranibizumab for polypoidal choroidal vasculopathy. Ophthalmology 2014;121:1151–2. 17. Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, et al. EVEREST study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–64.
18. Dugel PU, Jaffe GJ, Sallstig P, et al. Brolucizumab versus aflibercept in participants with neovascular agerelated macular degeneration: A randomized trial. Ophthalmology 2017;124:1296-1304.
19. Sridhar J, Hsu J, Shahlaee A. Topical dorzolamide-timolol with intravitreous anti-vascular endothelial growth factor for neovascular age-related macular degeneration. JAMA Ophthalmol 2016;134:4:437-43.
20.https://www.centerwatch.com/clinical-trials/listings/190529/neovascular-age-related-maculardegeneration-dorzolamide-timolol-combination-anti-vascular-endothelial/. Accessed 11 July 2018.



