A Yes. The 2017 Physician Fee Schedule Rule published in the Federal Register on November 15, 2016 is the second since the repeal of the Sustainable Growth Rate formula by the MACRA. The 2017 conversion factor is $35.8887, which is a slight increase from the 2016 conversion factor of $35.8043. It includes a budget neutrality adjustment of -0.013 percent, an increase of 0.5 percent resulting from MACRA and a misvalued code reduction target adjustment of -0.18 percent.
Q Are there significant changes to RVUs for ophthalmic services?
A Of the 553 CPT codes that apply to ophthalmology and optometry within the Medicare program, 526 of them changed very little: By three percent or less. Nine procedures had substantial increases in reimbursement; 18 had substantial reductions in reimbursement. Average change in Medicare reimbursement rates for ophthalmic services is miniscule (0.01 percent). Some retina and glaucoma procedure codes were favorably revalued after significant reductions in 2016. Others were reduced when the postop period was shortened to 10 days.
Q Is there a particular type of service that changes dramatically in 2017?
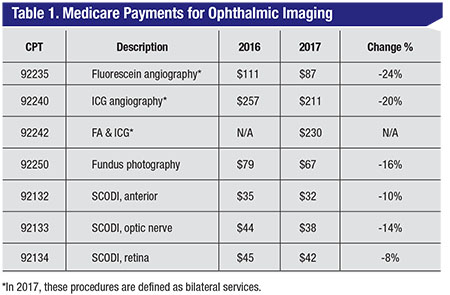
A Yes. In 2017, Medicare reimbursement for all ophthalmic imaging services is dramatically reduced (See Table 1). The redefinition of angiography as a bilateral service magnified the reduction. Formerly, angiography was paid per eye.
Q Will hospital outpatient departments and ambulatory surgery centers experience increases in facility reimbursement in 2017?
 |
A Various adjustments to hospital reimbursement result in a Hospital Outpatient Department rate increase of 1.65 percent. For 2017, the wage adjustment for budget neutrality in addition to the multifactor productivity-adjusted update factor increases the ASC conversion factor by 1.9 percent for those meeting the quality reporting requirements, resulting in small increases in facility reimbursement.
Q Are there new quality measures for ASCs to report in 2017?
A No. No new measures were added in 2017, which would affect payments in 2019. Seven additional measures were finalized for implementation in 2018, which will affect 2020 payments.
Q Did MACRA impose any new reporting requirements for surgeons?
A Yes. As of January 1, 2017, MACRA, Section 1848(c)(8)(B), requires collection of data to value global surgical packages including the number of and level of visits in the global period and other items and services related to surgery. The proposed rule contained onerous reporting requirements vehemently opposed by surgeons of all specialties. The final rule revises the reporting to include the following: submit with CPT code 99024; applies to groups with 10 or more practitioners; only applies in certain states (Florida, Kentucky, Louisiana, Nevada, New Jersey, North Dakota, Ohio, Oregon, Rhode Island); report services annually by more than 100 practitioners and more than 10,000 times or have allowed charges in excess of $10 million annually; can report beginning January 1, 2017; and mandatory reporting starting July 1, 2017.
The final list of codes will be posted to the CMS website; there are only 40 CPT codes that might potentially apply to ophthalmology.
Q What CPT code changes became effective on January 1, 2017?
A Category I CPT code changes are as follows:
New code:
∆ 92242 Fluorescein angiography and indocyanine-green angiography (includes multiframe imaging) performed at the same patient encounter with interpretation and report.
The following codes contain language changes described by underlines and represent clarifications and, in some cases, substantive revisions:
∆ 67101 Repair of retinal detachment, one or more sessions; cryotherapy or diathermy including drainage of subretinal fluid when performed; cryotherapy.
∆ 67105 Photocoagulation including drainage of subretinal fluid, when performed.
∆ 92235 Fluorescein angiography (includes multiframe imaging) with interpretation and report, unilateral or bilateral.
∆ 92240 Indocyanine-green angiography (includes multiframe imaging) with interpretation and report, unilateral or bilateral.
Deleted in 2017:
∆ 92140 Provocative tests for glaucoma, with interpretation and report, without tonography.
Q Are there any new Category III codes for 2017?
A Released semiannually by the American Medical Association, new Category III codes became effective January 1, 2017 following the six-month implementation period that began July 1, 2016.
∆ 0444T – Initial placement of a drug-eluting ocular insert under one or more eyelids, including fitting, training, and insertion, unilateral or bilateral.
∆ 0445T – Subsequent placement of a drug-eluting ocular insert under one or more eyelids, including re-training, and removal of existing insert, unilateral or bilateral.
∆ 0449T – Insertion of anterior segment drainage device, without extraocular reservoir; internal approach, into the subconjunctival space.
∆ +0450T – each additional device (List separately in addition to code for primary procedure).
Use 92499 For removal of aqueous drainage device without extraocular reservoir, placed into the subconjunctival space via internal approach.
Two new and one revised Category III codes are effective January 1, 2017 and will be published in the 2018 CPT manual:
∆ 0464T Visual evoked potential, testing for glaucoma, with interpretation and report.
For visual evoked potential screening for visual acuity, use 0333T.
∆ 0465T Suprachoroidal injection of a pharmacologic agent (does not include supply of medication.)
To report intravitreal injection/implantation, see 67025, 67027 or 67028.
∆ 0333T Visual evoked potential, screening of visual acuity, automated, with report.
Coverage and payment for Category III codes remains at the discretion of the Medicare Administrative Contractor.
Q What types of regulatory issues were identified in the Office of Inspector General’s annual work plan as areas of concern for ophthalmology in 2017?
A The annual publication of the Office of Inspector General’s Work Plan contains a few targets for scrutiny of concern to ophthalmologists and optometrists. The targets are:
• Drug Waste of Single-Vial Drugs (new);
• Management Review: CMS’ Implementation of the Quality Payment Program (new);
• ASC – Quality Oversight;
• Anesthesia services – Payments for personally performed services.
Q What Medicare Part B changes affect beneficiaries from a cost perspective in 2017?
A The Medicare Part B basic premium increases to $109 for most beneficiaries. The Part B deductible increases to $183. This is a $17 increase from the 2016 deductible.
Q What changes occurred with the Medicare Quality Programs?
A MACRA consolidates the current quality reporting programs of PQRS, EHR Meaningful Use and the Value-Based Payment Modifier into a new program: the Merit-Based Incentive Payment System. The law stipulates a January 1, 2017 start date, but the first bonus or penalty occurs in 2019 based on a two-year look-back. The penalties associated with the current quality reporting programs sunset after 2018.
Q Were changes made to the electronic health record Meaningful Use reporting?
A Yes. The 2015 Electronic Health Record Flexibility Rule revised the requirements for Meaningful Use attestation. For 2015 to 2017, all eligible professionals must report on 10 mandatory objectives included in the Modified Stage 2 Rule. Failure to attest for 2016 participation results in a 4-percent penalty in 2018. Providers who have already attested to MU in prior years are required to report MU for any continuous 90-day period in 2016 and 2017. The reporting deadline for 2016 is February 28, 2017. If a provider has never attested to MU and is attesting for the first time, attestation by October 1, 2017 avoids a 2018 penalty. REVIEW
Ms. McCune is vice president of the Corcoran Consulting Group. Contact her at DMcCune@corcoranccg.com.



