In recent years, there’s been a lot of excitement about minimally-invasive glaucoma surgery. It’s important, however, to not let all that buzz eliminate thoughts of other, alternative procedures as you diagnose a patient’s glaucoma status and choose the best treatment. Sometimes, the best procedure for a patient with moderate to advanced glaucoma isn’t MIGS; the time-tested options of tube shunts and trabeculectomy are still vital parts of our surgical arsenal and, in these types of cases, may be the better choice.
MIGS’ Shortcomings
The heart of the matter is that the amount of intraocular pressure reduction achieved by MIGS is often not impressive, though the procedures have proven to be safe overall. A lot of the IOP lowering efficacy of these procedures is likely the result of their being combined with cataract surgery. When I’m discussing the various surgical options with my glaucoma patients, I often use a baseball analogy: MIGS can be viewed as a hitter who doesn’t hit home runs, but instead routinely hits singles and occasionally doubles with very few strikeouts.
In addition to modest amounts of pressure reduction (which will most likely leave patients on one or two glaucoma medications postoperatively), MIGS has the limitation of needing to be combined with cataract surgery in order for the surgeon to be reimbursed. (Note: The XEN gel stent doesn’t necessarily need to be combined with cataract surgery, but it functions similarly to a modified shunt device, so I view it slightly differently than a conventional cataract surgery-associated MIGS procedure.) This proves to be problematic in patients with glaucoma who have already had cataract surgery—you may not be able to perform a MIGS procedure and have the surgery covered by insurance.
On the alternative side is the patient with glaucoma who has minimal/no cataract, but requires significant IOP reduction. In my opinion, rendering the patient pseudophakic while she’s still capable of accommodation is dreadful. If a patient has a clear lens, it doesn’t make sense to me to just take it out in order to perform a MIGS procedure. Also, uncomplicated cataract surgery isn’t without its own inherent risks, such as an increased long-term risk of retinal detachment in a high myope. Just recently, I saw a fairly young patient with glaucoma who developed a rhegmatogenous retinal detachment, and his only apparent risk factors were his mild myopia and history of uneventful cataract surgery two years ago.
MIGS also adds costs to the cataract procedure, given the not-insignificant costs of the MIGS devices. These procedures also involve a non-trivial learning curve, new for each MIGS device; in contrast, most glaucoma surgeons have received superb training in trabeculectomy and tube shunt placement.
In my opinion, MIGS is also mostly reserved for the garden-variety, primary open-angle glaucoma patient with mild disease, while trabeculectomy and tube shunts can be used in a wider array of glaucomas, such as chronic angle closure, neovascular and inflammatory glaucomas. The jury is still out on MIGS’ efficacy in these other glaucoma diagnoses.
Thus, “hitting singles and doubles” with MIGS may be acceptable for certain subsets of patients who can tolerate possibly being on one or more medications after the surgery. But sometimes, you, as the surgeon, need to bring in the heavy hitters.
Batter Up
If a modest pressure reduction isn’t enough for the patient, it’s time to consider trabeculectomy or a glaucoma tube shunt.
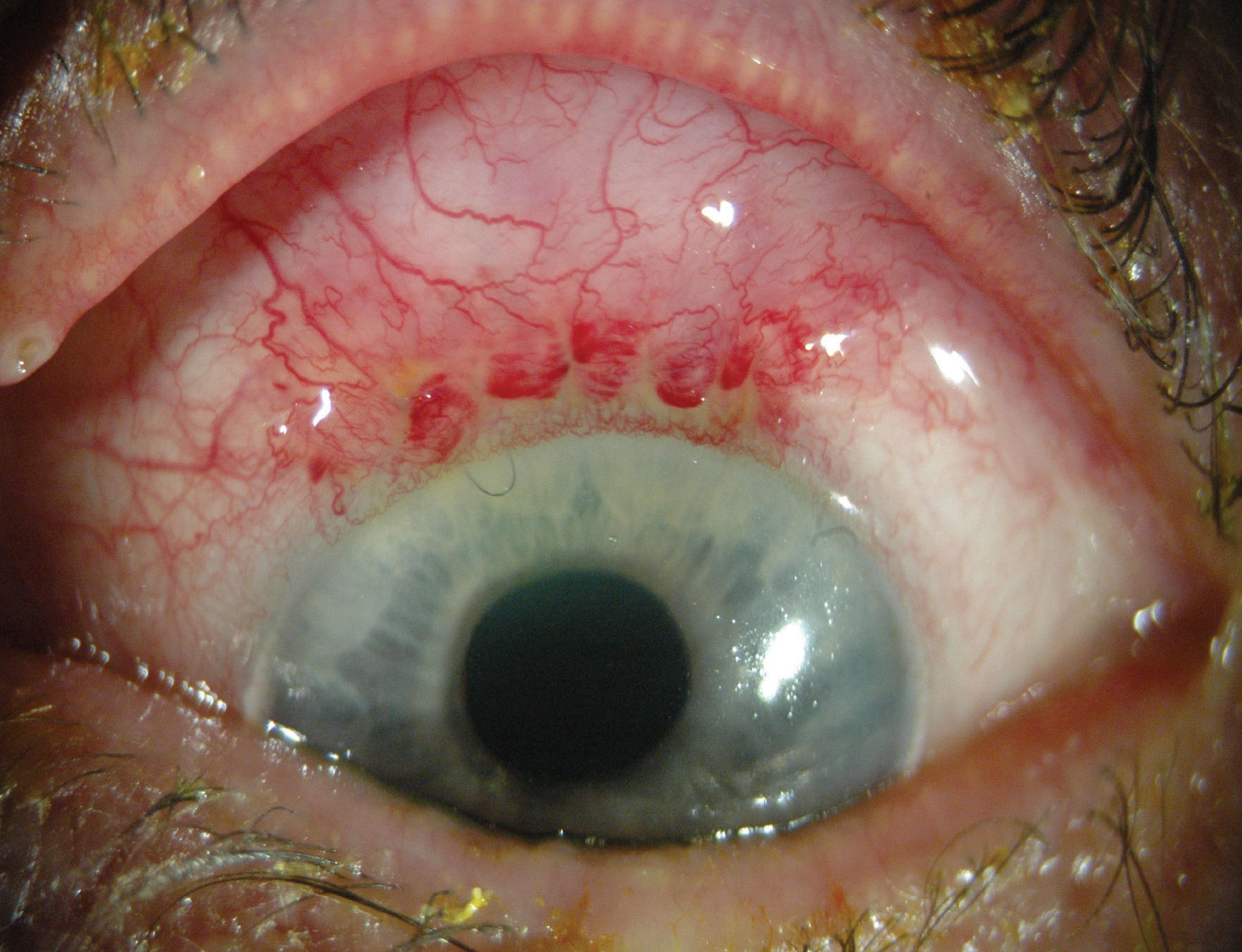
• Trabeculectomy’s advantages. For those patients who need significant pressure reduction, this usually means a reduction in IOP of 30 percent or more, a target level often times out of MIGS’ average reach. Trabeculectomy, however, is capable of delivering this amount of IOP lowering efficacy, and at least one study, the Collaborative Initial Glaucoma Treatment Study, demonstrated that trabeculectomy reduced pressures by approximately 48 percent from baseline readings at three years.1 CIGTS set an aggressive minimum IOP target lowering goal for its patients—at least 30 percent—and, in addition to the 40-percent reduction with trabeculectomy (in the five-year results)—it also reported that medication achieved 35-percent reduction. Thus, for a patient who requires significant pressure reduction, the treatment has to at least be equivalent to the medication arm of CIGTS. Consider a patient who has an uncontrolled pressure of 21 mmHg, and is intolerant of most if not all medications; if I’ve set a target pressure goal in the low teens, I’ll turn to trabeculectomy. When speaking to a patient with a low target IOP range and a need for sustained long-term IOP reduction, I envision that most MIGS procedures would not sufficiently lower IOP into the low teens even with supplemental medications (i.e. a single drop). With trabeculectomy, on the other hand, you actually have a chance of hitting a home run— great pressure reduction, a healthy appearing, non-ischemic filtering bleb; and a delighted patient who is no longer taking any medications.
 |
|
Also, since trabeculectomy doesn’t involve placing any foreign materials (besides sutures), there’s no need to worry that a device will shift its position and/or migrate to a different location. In a similar vein, since trabeculectomy doesn’t involve a device, but instead uses intrinsic ocular tissue to achieve its effect, the procedure is extremely cost-effective. Incidentally, this is one of the reasons some surgeons prefer MIGS such as the Kahook Dual-Blade and the Trabectome, since these procedures don’t involve placing materials into the trabecular meshwork or placing a transscleral device like the XEN. In addition, a foreign object can become plugged and/or erode through adjacent tissue.
• Trabeculectomy’s downsides. The reason, however, we don’t place the home-run hitter at the plate in every situation is that along with that potential for the game-changing home run comes the increased risk of striking out.
While MIGS may not be as effective in lowering IOP as trabeculectomy, the former has a lower risk profile, while the latter is associated with such complications as hypotony, bleb infections and suprachoroidal hemorrhage. The Primary Tube vs. Trabeculectomy Study illustrates this point: “Lower IOP with use of fewer glaucoma medications was achieved after trabeculectomy with MMC compared with tube shunt surgery during the first year of follow-up. The frequency of serious complications producing vision loss or requiring reoperation was lower after tube shunt surgery relative to trabeculectomy with MMC.”2 Also, it’s worth remembering that the type of endoph--thalmitis associated with trabeculectomy is a more devastating intraocular infection than that which occurs following routine cataract surgery. In trabeculectomy-associated endophthalmitis, the bacteria tend to be more virulent.
Because of these inherent risks, you, as the surgeon, need to choose your trabeculectomy patient very carefully. For example, if the potential patient is in a nursing home and not easily accessible for postoperative follow-up visits (and has a limited support system) trabeculectomy is not an ideal operation, given the higher rates of infection and/or bleb-related complications.
Additionally, most of us who perform trabeculectomies also use adjunctive mitomycin-C. With mitomycin-C, however, there’s an increased risk of bleb avascularity, with an increased risk of bleb leaks that can lead to blebitis and endophthalmitis.
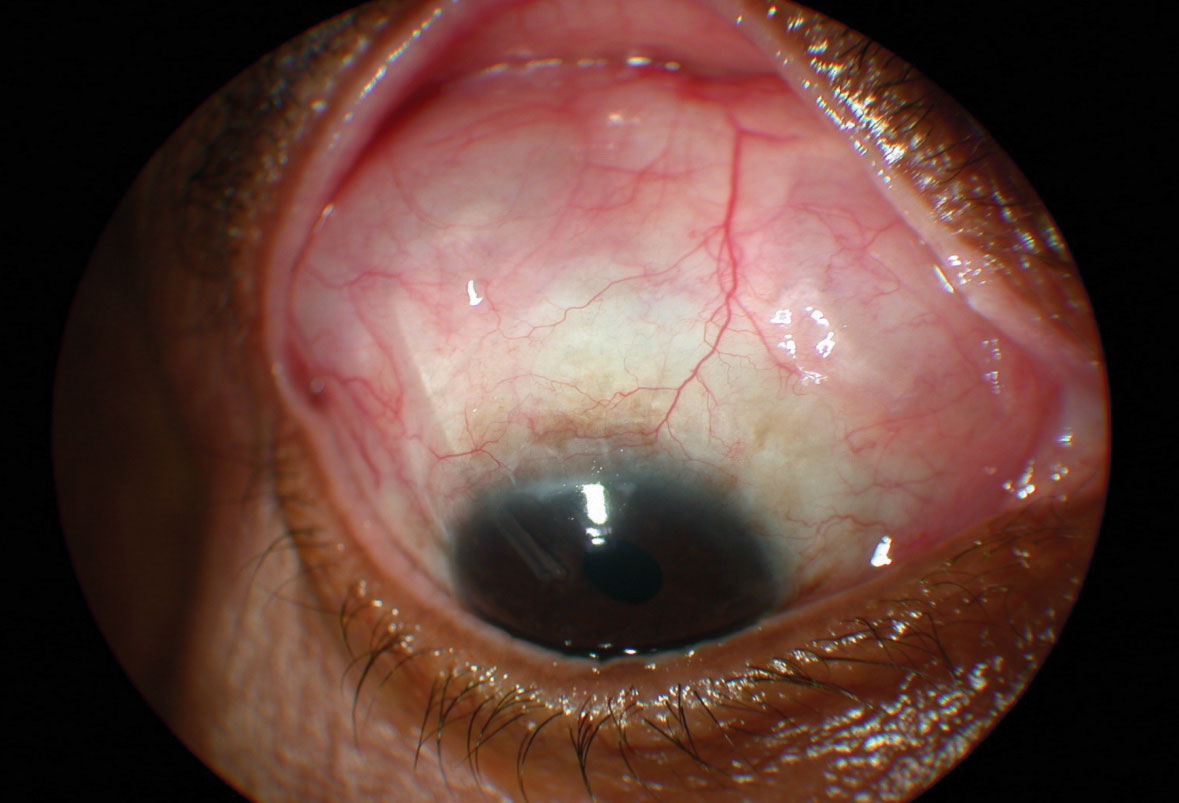
• Glaucoma tube shunts’ advantages. When discussing tube shunts with patients and using the baseball analogy, I tell the patients that these procedures are positioned somewhere between the modest base hits of MIGS and the home run potential of trabeculectomy. In my opinion, glaucoma tube shunt implants function more like consistent doubles hitters, with less risk for the bad strikeouts associated with trabeculectomy. After successful tube shunt surgery, on average, the patient is usually on at least one or two medications and his/her IOP control may not be quite as good as following trabeculectomy. However, the results of tube shunt surgery are usually more consistent than those following trabeculectomy. Even though, admittedly, most tube shunt results are surgeon-dependent, by and large I believe that these surgeries have less variability in their outcome than does performing a trabeculectomy.
 |
|
|
Along with this consistency, if not home-run ability, comes fewer “strikeouts,” which translates into a much lower risk of endophthalmitis than trabeculectomy. There’s still a risk of suprachoroidal hemorrhage with a tube shunt, but you can reduce this risk by performing the tube shunt very carefully.
Supporting this idea, the cumulative probability of failure during five years of follow-up in the Tube vs. Trabeculectomy Study was 29.8 percent in the tube group and 46.9 percent in the trabeculectomy group (p=0.01). In terms of efficacy, the IOP result was 14.4 ±6.9 mmHg in the tube group and 12.6 ± 5.9 mmHg in the trabeculectomy group at one year (p=0.01), and the number of glaucoma medications was 1.4 ±1.3 in the tube group vs. 1.2 ±1.5 in the trabeculectomy group (p<0.001).3
When selecting the best patient for tube shunt surgery, look for a patient who might have inconsistent follow-up during the immediate postop period; tube shunts involve less postoperative manipulation than trabeculectomy. The latter procedure can manifest with very high or low pressures postoperatively depending on the condition of the bleb, and your subsequent postop manipulation. A good surgical candidate is also someone who doesn’t need a super-low target pressure. The type of glaucoma also comes into play: Trabeculectomy would be the surgery of choice in patients with normal- or low-tension glaucoma who need significant IOP reduction, whereas a tube shunt is better for patients with neovascular glaucoma, because it seems to work better than a traditional trabeculectomy with an antimetabolite in these types of patients. Also, someone with refractory glaucoma and/or one of the more complicated glaucoma presentations, or a history of glaucoma or other ocular surgery—especially a failed trabeculectomy—would be a good candidate for a tube shunt.
• Tube shunt disadvantages. Though the tube shunt is a good middle-of-the-road option between MIGS and trabeculectomy, it’s not without complications. Because the procedure involves the introduction of a foreign body, one of the main complications is extrusion of the implant, in which the tube erodes through either the patch graft or a scleral flap. There’s also the aforementioned risk of suprachoroidal hemorrhage and endophthalmitis, but the latter occurs at a lower rate than with trabeculectomy.
 |
A tube implant may not be a good option in a patient with corneal endothelial problems, because there’s always the concern of endothelial problems with a tube in the anterior chamber; for example, if the patient rubs his eye, the tube might contact the corneal endothelium. The other type of patient who isn’t an ideal candidate is one who can’t tolerate having a foreign body sensation under the lid (patients have sometimes reported feeling an uncomfortable sensation of “feeling the implant”).
In the end, it’s best if you as a glaucoma surgeon don’t focus on just one type of procedure, be it MIGS, trabeculectomy or tube shunts. If you only perform one type of procedure, I believe it will be rather difficult to have excellent surgical outcomes when treating all the different types of glaucoma patients. Instead, having a breadth of surgical skills and experience will allow you to recommend the surgery that’s most appropriate for a particular patient, rather than trying to make one surgery fit all clinical indications. REVIEW
Dr. Tsai is president of New York Eye and Ear Infirmary of Mount Sinai, as well as Delafield-Rodgers professor and system chair of ophthalmology at the Icahn School of Medicine at Mount Sinai.
1. Feiner L, Piltz-Seymour JR; Collaborative Initial Glaucoma Treatment Study. Collaborative Initial Glaucoma Treatment Study: A summary of results to date. Curr Opin Ophthalmol 2003;14:2:106-11.
2. Gedde SJ, Feuer WJ, Shi W. Treatment outcomes in the Primary Tube Versus Trabeculectomy Study after 1 year of follow-up. Ophthalmology 2018;125:5:650-663.
3. Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol 2012;153:5:789-803.



