This patient with gradual, painless, blurred vision OD was found to have a unilateral hyperopic shift of 1.5 D from an underlying choroidal mass.
The differential diagnosis for a unilateral hyperopic shift includes mechanisms that flatten the cornea, shift the lens posteriorly, or shift the retina anteriorly.
In this case, there was an amelanotic choroidal mass that shifted the retina anteriorly. Differential diagnosis included choroidal metastasis, choroidal nevus, choroidal melanoma, choroidal hemangioma, choroidal granuloma, choroidal lymphoma, posterior scleritis and Harada’s syndrome.
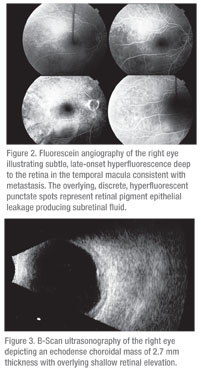
The patient was referred to the Wills Eye Institute Ocular Oncology Service, where examination, fluorescein angiography and ultrasonography were performed (See Figures 2 and 3). The fluorescein angiography depicted a round choroidal mass with overlying punctate hyperfluorescent foci, suggestive of a choroidal tumor with retinal pigment epithelial leaks producing subretinal fluid. The B-scan ultrasound showed a 12 x 11 x 2.7-mm echodense choroidal mass.
The clinical history and features were suggestive of choroidal metastasis. Further questioning revealed a history of breast cancer five years prior with metastases to the lung and bone. He had undergone mastectomy, chemotherapy, radiation, and Tamoxifen therapy and was in remission for the previous three years.
The patient was found to have other sites of active metastasis in the lung and bone marrow as well. Treatment with systemic chemotherapy agents was planned. Given the progressive visual loss, local plaque radiotherapy for the choroidal metastasis was performed.
By six months follow-up, the metastasis had regressed to a flat atrophic scar and visual acuity was 20/300. Further small choroidal metastases were detected in each eye and the patient was continued on chemotherapy.
 Discussion
Discussion
Breast cancer is the most common malignancy found in women, accounting for 27 percent of newly diagnosed cancers in women. In 2009 in the United States, there were approximately 190,000 cases of breast cancer in women and 1,900 in males. Breast cancer in males is exceedingly rare. Of all cases of breast cancer in the United States, <1 percent occur in males.1,2
The pathogenesis, risk factors, genetics, clinical findings and treatments are similar in males and females, but there are important differences as well. Fifty percent of males have Stage II or greater breast cancer at the time of diagnosis, as compared to 35 percent of females. Additionally, only 5 percent of males had HER2/neu positive cancer as compared with 15 percent of females. African-American males are more likely to get breast cancer than Caucasian males, whereas Caucasian females develop breast cancer more frequently than African-American females.
Risk factors for the development of breast cancer in males include the presence of or family history of BRCA1 or BRCA2 mutations, a history of estrogen exposure or androgen insufficiency, exposure to radiation and occupational exposure to high heat environments.
Males at known increased risk are recommended to perform monthly personal breast examination and yearly screening mammography. Treatment of male breast cancer is mostly based on known treatment modalities and algorithms for female breast cancer.3
The uvea is the most common site for ocular metastasis. A comprehensive survey of uveal metastases seen on the Ocular Oncology Service at Wills Eye Institute included 520 eyes of 420 patients with a total of 950 uveal metastases. The authors reviewed systemic and ophthalmic features of uveal metastases. The most common primary cancer sites for uveal metastasis in males were lung (40 percent), gastrointestinal (9 percent), kidney (8 percent), and other (including breast in 1 percent). In females, the most common sites included breast (68 percent), lung (12 percent) and other (4 percent).
Within the uvea, 88 percent of metastases are to the choroid, followed by metastases to the iris (9 percent) and ciliary body (2 percent). Choroidal metastases are typically yellow in color (in 95 percent of lesions), have a plateau configuration and are associated with subretinal fluid. Patients most often present with blurred vision (70 percent), flashes and floaters (12 percent) and pain (7 percent). It is not uncommon for metastases to be found in asymptomatic patients (11 percent).4
Breast cancer is the most common cancer to metastasize to the choroid. In a study of 264 patients with uveal metastases from breast cancer, 62 percent of patients had unilateral metastasis at presentation. Patients with breast cancer metastatic to the uvea show survival rates of 65 percent at one year, 35 percent at three years and 24 percent at five years.5
A thorough history, physical exam, laboratory evaluation and imaging evaluation will rule out other systemic metastases. Fine needle aspiration biopsy (FNAB) can be used to confirm the diagnosis of metastasis if primary cancer is unknown. There are few risks associated with FNAB. In a study of 159 FNABs for suspected intraocular tumors, the most common complication was subretinal hemorrhage in 13 percent of patients.6
Treatment for choroidal metastases includes systemic chemotherapy and/or hormonal therapy as well as plaque radiotherapy, external beam radiation therapy and/or photodynamic therapy.
Plaque radiotherapy is usually reserved for solitary metastases. The average life expectancy for patients with choroidal metastases is less than one year.
Complications from plaque radiotherapy are similar to those of external beam and other radiation therapies, including radiation retinopathy, papillopathy, and cataract. These side effects are uncommon, especially given the short life expectancy of many patients.7
The author would like to thank Carol Shields, MD, Wills Eye Institute Ocular Oncology Department, for her time and assistance.
1. American Cancer Society: Cancer Facts & Figures 2009-2010. http://ww2.cancer.org/downloads/STT/500809web.pdf. Accessed 01-23-2011.
2. American Cancer Society: Breast Cancer Facts & Figures 2009-2010. http://ww2.cancer.org/downloads/STT/F861009_final percent209-08-09.pdf. Accessed 01-23-2011.
3. Johansen Taber KA, Morisy LR, Osbahr III AJ, Dickinson BD. Male breast cancer: Risk factors, diagnosis, and management (Review). Oncology Reports 2010;24:1115-1120.
4. Shields CL, Shields JA, Gross N, Schwartz G, Lally S. Survey of 520 eyes with uveal metastases. Ophthalmology 1997;104:1265-76.
5. Demirci H, Shields CL, Chao A-N, Shields JA. Uveal Metastasis from Breast Cancer in 264 Patients. Am J Ophthalmol 2003;136,2:264-71.
6. Shields JA, Shields CL, Ehya H, Eagle RC, Jr, DePotter P. Fine needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick Lecture. Ophthalmology 1993;100:1677-1684.
7. Shields CL, Shields JA, De Potter P, Quarante M, Friere J, Brady LW, Barrett J. Plaque radiotherapy for the management of uveal metastasis. Arch Ophthalmol 1997;115:203-9.



