Diagnosis, Workup and Treatment
The patient was diagnosed with mild panuveitis OD, likely secondary to toxoplasmosis. The following laboratory workup was initiated: toxoplasma titers; chest x-ray; PPD with anergy panel; FTA-ABS; RPR; toxocara titer; ESR; ANA; Lyme assay; CBC with differential; and HIV and blood cultures. The patient was observed without treatment. Her toxoplasma IgG titer was positive at 4.90 (normal <1.10). Her toxoplasma IgM was negative, and the remaining workup was negative.
Discussion
Toxoplasmosis is a protozoan with an impressive curriculum vitae. Positive titers are found in 23 percent of U.S. citizens, 67 percent of pregnant Parisians, and in 98 percent of individuals living in certain regions of southern Brazil. It is the third deadliest food-born pathogen in the United States and has both sexual and asexual capabilities.
The feline is the definitive host. Oocysts are shed in the cat feces and can be ingested from contaminated water, unwashed vegetables or cat litter. Humans may acquire toxoplasmosis from consumption of undercooked meats such as beef or lamb containing toxoplasma cysts.
|
Pregnant women who have not been previously exposed to toxoplasma may acquire the infection, which can cause injury to the fetus. Congenital infection occurs if the mother has never been exposed to toxoplasmosis. Once a woman has been exposed to toxoplasmosis, she will form anti-toxoplasma IgG, which will protect all subsequent offspring.
Toxoplasmosis is thought to be the most common retinal infection. Historically, most infections were thought to be congenital; however, current thinking suggests that up to two-thirds of ocular toxoplasmosis is acquired after birth. Eighty percent of congenitally infected humans will have ocular findings. If acquired after 13 years of age, 2 percent of individuals in the United States will have ocular findings in contrast to 21 percent of southern Brazilians. This disparity is due to the different strains of toxoplasmosis found throughout the world. In Brazil, type I toxoplasmosis is predominant, and it is much more virulent than the type II and III strains found in the United States.
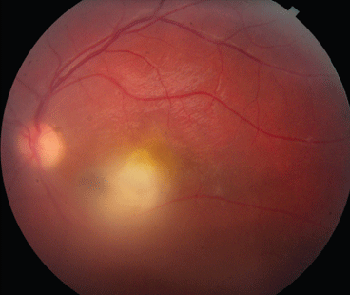
Findings of ocular toxoplasmosis include well-circumscribed, dark chorioretinal scars representing old, inactive disease or active lesions ranging from large, destructive, full-thickness lesions to deeper punctate lesions. Typical lesions are thick, yellow-white with contoured edges, not associated with hemorrhage. There is often an overlying vitritis that in more advanced cases can give the classic "headlight in a fog" appearance. Often there is one focus at the border of an old scar where multiple recurrences occur, suggesting a nidus of reactivating cysts. Sixty percent of congenital lesions occur in the macula versus 38 percent of acquired lesions. This disproportionate affinity for the macula possibly relates to the lack of macrophages in the macula. Visual loss in ocular toxoplasmosis is most often secondary to vitritis. However, visual loss may also be secondary to lesions occurring in the macula or on the optic nerve head, which may cause permanent vision loss. With lesions larger than one disk diameter, the risk of vision loss, retinal detachment, cataract, chronic inflammation, glaucoma and macular edema increases.
Treatment of ocular toxoplasmosis remains highly controversial without evidence-based protocol. Most agree that treatment should be given in cases of vision-threatening lesions of the macula or optic nerve and in immunocompromised patients. Some combination of pyrimethamine (+ folinic acid), sulfadiazine, trimethoprim/sulfamethoxazole, atovaquone or clindamycin in combination with steroids is the mainstay of treatment. Steroids should not be given alone or to the immunocompromised. Physicians should be aware that these drugs are not without risk. For example, sulfa drugs can cause Stevens-Johnson syndrome, and clindamycin has been associated with Clostridium difficile colitis. If the lesions are outside of the posterior pole and the vitritis is not severe, the lesions can be observed.
Thus far, only three prospective, randomized, placebo-controlled, clinical trials have been conducted evaluating the treatment of ocular toxoplasmosis. The use of pyrimethamine as well as pyrimethamine-trisulfapyrimidine with oral steroids for acute infection, and prophylactic trimethoprim-sulfamexacocol for recurrent retinochoroiditis was studied. All of the studies were considered to be methodologically poor upon Cochrane evaluation, and none reported on long-term visual acuity. There was no benefit found for treating acute infection, and there was only weak benefit found with treatment of recurrent disease when instituted for greater than 14 months. Two of the studies found decreased inflammation with treatment; however, increased drug adverse events occurred in two studies.
Follow-up and Conclusion
On follow-up the next day at the Wills Eye Institute Retina Service, the patient's vision had improved to 20/40 OD. Triple therapy was begun; however, there was disagreement among the retina faculty as to whether this was necessary. Further follow-up occurred out of state.
In retrospect, the patient's initial ER workup was thought to be unnecessary given the patient's classic presentation.
In conclusion, toxoplasmosis is a ubiquitous disease with high rates of ocular involvement. Treatment remains controversial given the relative lack of long-term randomized controlled trials and the potential risks associated with the medications used for treatment. REVIEW
Dr. Cropsey would like to thank Sunir Garg, MD, Wills Eye Institute Retina Service, for his assistance with this case.
Gilbert RE, Harden M and Stanford MR. Antibiotics versus control for toxoplasma retinochoroiditis. Cochrane Database of Systematic Reviews 2006;4.
Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol 2003;136:973-88
Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. Am J Ophthalmol 2004;137:1-17.
Koo L, Young LH. Management of ocular toxoplasmosis. Int Ophthalmol Clin 2006;46:183-93.
Kump LI, Androudi SN and Foster CS. Ocular toxoplasmosis in pregnancy. Clin Experiment Ophthalmol 2005;33:455-60.