 |
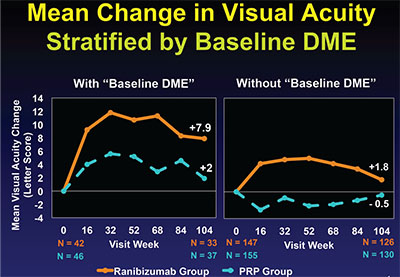
| Figure 1. Mean changes in visual acuity at two years in Protocol S in PDR eyes with and without baseline DME. The difference in eyes with baseline DME (left) significantly favors the ranibizumab treatment arm over the panretinal photocoagulation arm, despite both groups receiving ranibizumab injections for DME. The difference in the PRP arm (right) is not significant and met the non-inferiority limit for ranibizumab not being worse than PRP. All figures provided and copyright owned by the DRCR Network. |
Protocol S
To understand the significance of Protocol S, it helps to discuss PDR and how we’ve treated it in the past. Untreated, PDR is a leading cause of blindness in the diabetic population. PRP has been an effective but inherently destructive treatment for four decades. Multiple trials of anti-vascular endothelial growth factor therapy for DME have reproducibly demonstrated significant regression of retinopathy severity with VEGF inhibition.4-6 This observation led to considerable interest in comparing PRP to anti-VEGF therapy for preventing sight-threatening complications of PDR.
In Protocol S, the primary objective was to compare the safety and efficacy of PRP to intravitreous ranibizumab 0.5 mg injections for the treatment of PDR (the 0.5-mg dose was used because at study initiation, the 0.3-mg dose was not yet Food and Drug Administration-approved or available). The primary outcome of the study was that ranibizumab was non-inferior to PRP, with a non-inferiority limit of five letters.
Important secondary outcomes included an area-under-the-curve (AUC) analysis of vision, changes in visual field, development of DME and rates of vitrectomy for complications such as vitreous hemorrhage and traction retinal detachment.
At 55 sites, researchers randomized 394 eyes of 304 patients with active PDR, vision better than or equal to 20/320 and no prior PRP in a 1:1 ratio to receive either six injections of ranibizumab 0.5 mg at monthly intervals (n=191), or immediate PRP in one to three sessions over a maximum eight-week period (n=203). In the ranibizumab group, subsequent injections were given according to a prespecified retreatment algorithm over the initial two years of follow-up, and supplemental PRP was given in the laser group according to similar criteria. The retreatment decision rested on whether the neovascularization of the disc/neovascularization elsewhere was resolved, persistent but stable, or worsening. Center-involving DME at baseline was allowed in both groups and researchers treated it with ranibizumab injections using the DRCR retreatment algorithm from Protocol I.7
At baseline, the groups were well balanced in respect to age, sex, duration of diabetes, mean A1C level and no prior PRP. Mean baseline vision was 75 letters (20/32) in both groups, and the mean OCT central subfield thickness was 262 in the ranibizumab group and 249 in the PRP group. Center-involving DME was present at baseline in 22 percent and 23 percent of eyes in the ranibizumab and PRP groups, respectively.
In the ranibizumab group, eyes with baseline DME (n=36) received a median of 14 injections over two years versus a median of 10 injections in eyes without baseline DME (n=133). Only 6 percent of ranibizumab eyes received PRP for rescue treatment over two years, usually during vitrectomy. In the PRP group, 98 percent of eyes received initial PRP according to protocol, and 45 percent received supplemental PRP for worsening PDR at a median of seven months from baseline. In the PRP group, 55 percent of eyes received at least one ranibizumab injection for DME during the two years of follow-up. PRP eyes with baseline DME (n=42) received a median of nine injections, while eyes without baseline DME (n=135) received a median of zero injections through two years.
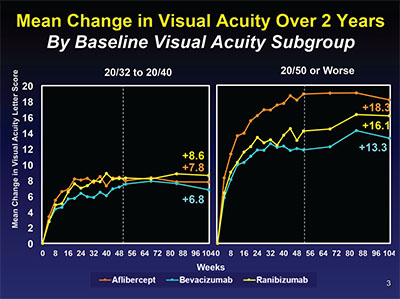 |
| Figure 2. Mean changes in visual acuity by baseline vision subgroup of 20/32 to 20/40 on the left and 20/50 to 20/320 on the right. The difference between aflibercept and ranibizumab seen at one year in the worse vision group wasn’t seen at two years. |
At the two-year primary endpoint, ranibizumab was non-inferior to PRP with a mean gain of +2.8 letters vs. +0.2 letters in the PRP group. This met the non-inferiority limit of -5 letters. In eyes with central DME at baseline, ranibizumab gave superior improvement in visual acuity with a gain of eight letters versus two letters in the PRP group, despite both arms receiving ranibizumab injections for the DME (Figure 1).
Important secondary outcomes also favored ranibizumab. Visual field loss, measured in decibels by Humphrey analyzers using the 30-2 and 60-4 programs, was significantly less in the ranibizumab arms, with a mean of -23 decibels lost versus -422 decibels in the PRP arm. The pre-planned AUC analysis favored ranibizumab +4.5 letters vs. +0.3 letters for the PRP group. Not surprisingly, given the known effect of ranibizumab on DME, eyes in the ranibizumab group had greater reductions in optical coherence tomography CST, and fewer eyes developed DME during the two years of the study (9 percent vs. 28 percent in the PRP group). Also, rates of vitrectomy were less at 4 percent, vs. 15 percent in the PRP group. Fortunately there were no significant differences between the two treatment arms in ocular or systemic safety events.
So protocol S clearly demonstrated that ranibizumab is at least as effective as PRP in treating PDR (though in both groups about 40 to 45 percent of eyes had active NV at two years). There’s significant data that ranibizumab is a better treatment, with superior two-year visual acuity gains, particularly in eyes with baseline DME, and dramatically less visual field loss compared to PRP. Additionally, ranibizumab treated eyes were less likely to develop DME and less likely to require vitrectomy.
However, there are important differences in treatment burden, cost and risk of worsening disease if a patient treated with ranibizumab fails to return for appropriate follow-up. PRP has a proven track record of long-term stability of PDR regression, with most eyes remaining stable for as long as 15 years after laser with no additional treatment.8 The two-year outcomes thus far don’t allow us confidence in the long-term stability of PDR treated with ranibizumab alone. The study is planned for five years, so those ongoing results will inform us as we integrate this data into our clinical practice.
Finally, I believe the most important outcome of this study is the superior vision outcomes with ranibizumab in eyes with PDR and central DME at baseline. Remarkably, eyes in the PRP group that had baseline DME would have received ranibizumab according to the same retreatment algorithm as eyes with DME in the ranibizumab group, yet showed significantly less improvement in vision, suggesting a negative effect of PRP on vision. When adding the benefit to visual field, ranibizumab treatment would seem to be the preferred approach in these eyes.
Protocol T
The DRCR Protocol I results, published in 2010, demonstrated the superior visual improvements in eyes with center-involved DME treated with intravitreous ranibizumab 0.5 mg, with prompt or deferred focal/grid laser, over laser alone and intravitreous triamcinolone plus focal/grid laser.9 This established ranibizumab as the preferred therapy for center-involved DME. Prior to the FDA approval of ranibizumab 0.3 mg for the treatment of DME, bevacizumab 1.25 mg was being widely used off label as a substitute for ranibizumab to treat DME. Similar to the situation with neovascular AMD and the Comparison of AMD Treatments Trials, the DRCRnet organized Protocol T, a non-inferiority study to compare ranibizumab and bevacizumab for DME. Prior to study initiation, aflibercept 2 mg became FDA approved for neovascular AMD treatment. With aflibercept available, the study was changed to a three-way superiority study. The investigators adjusted the ranibizumab dose to 0.3 mg after the FDA approval of that dose for DME in September 2012, shortly after the study began.
In protocol T, 660 eyes with visual acuity of 20/32 to 20/320 and center-involving DME on clinical exam, confirmed by OCT, were enrolled at 89 sites. No prior anti-VEGF therapy in the previous 12 months was allowed, as well as no laser or steroid treatment for DME in the prior four months. The treatment groups were intravitreous injections of aflibercept 2 mg (n=224), bevacizumab 1.25 mg (n=218) or ranibizumab 0.3 mg (n=218). At baseline, the three groups were comparable in terms of visual acuity (68 to 69 letters) and mean OCT central subfield thickness (376 to 390 µm). There was no difference in the percentage of eyes with prior laser for DME (36 to 39 percent) or with prior anti-VEGF therapy (11 to 14 percent). There were also no imbalances in systemic baseline characteristics such as age, duration of diabetes, race or hemoglobin A1C values. Retention in the study was excellent, with more than 95 percent of patients completing the one-year primary outcome visit and more than 90 percent completing the two-year final visit.
Starting at the baseline visit, injections were given as often as every four weeks according to a prespecified retreatment algorithm that was Web-based and determined in real time during the study visit. Essentially, in the first year patients were examined every four weeks and, after the baseline injection, retreatment was given if the eye improved or worsened in vision by five or more ETDRS letters or in OCT CST by 10 percent or more. In the first six months, injections were continued unless the eye achieved 20/20 or better vision and the OCT CST became normal (250 µm or less Stratus equivalent).10 At or after six months, if the vision and OCT were stable for two consecutive injections and there was persistent edema or vision loss, investigators applied focal grid laser and stopped injections. After that, if the vision or OCT worsened, they resumed the injections. This implies that the protocol tolerated the persistent edema and vision loss after six months and non-protocol treatment for persistent edema wasn’t allowed.
In the second year, visits could be extended up to 16 weeks depending on the status of the eye; retreatment in year two was only allowed if the vision or OCT CST worsened. Compliance with protocol treatment was high through two years: Researchers gave 98 percent of required injections, and injections were given when the protocol indicated deferral in only 0.5 percent of visits. Non-protocol alternative treatments were given in three eyes (1 percent), 10 eyes (5 percent) and 1 eye (<1 percent) in the aflibercept, bevacizumab and ranibizumab groups, respectively, that met treatment failure criteria.
In the first year, eyes received a median of nine, 10 and 10 injections in the aflibercept, bevacizumab and ranibizumab groups, respectively. In the second year, the number of injections declined by approximately 40 percent, to a median of five, six and six, in the aflibercept, bevacizumab and ranibizumab groups, respectively. The total number of injections was essentially equivalent across the three groups: 15; 16; and 15. So there was no difference in treatment burden between agents.
The decision to apply focal/grid laser was driven by the presence of persistent central DME at six months, and there was a benefit in the aflibercept group compared to the other two agents throughout the two years of the study. In both years, and overall, eyes in the aflibercept group were less likely to require laser. At the end of two years, 41 percent, 50 percent and 64 percent of eyes in the aflibercept, ranibizumab and bevacizumab groups, respectively, received at least one laser treatment, which was statistically significant for all three pairwise comparisons.
The primary outcome of the study was the mean change in visual acuity from baseline at one year. The study was planned for two years to examine longer-term outcomes. For the overall comparison, aflibercept was superior to the other two agents, with a mean of +13.3 versus +11.2 versus +9.7 letters gained for the aflibercept, ranibizumab and bevacizumab groups, respectively. But in year two, the difference between aflibercept and ranibizumab disappeared, while bevacizumab remained inferior to aflibercept but not ranibizumab. But this overall comparison has little clinical utility because there was a very strong interaction with baseline visual acuity.
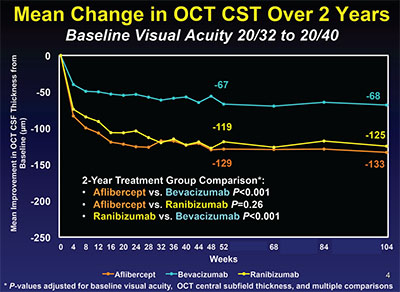 |
| Figure 3. Through two years in Protocol T, bevacizumab-treated eyes had about half as much reduction as the other two treatment groups. |
In eyes with better baseline vision of 20/32 to 20/40, there was no significant difference between the three agents at one and two years. But in eyes with baseline vision 20/50 or worse, eyes in the aflibercept group gained significantly more vision than either ranibizumab- or bevacizumab-treated eyes. However, in year two, the difference between aflibercept and ranibizumab narrowed and was no longer statistically significant. Bevacizumab remained inferior to aflibercept but not ranibizumab (Figure 2, p. 78).
Gaining three or more lines of vision has historically been considered a clinically significant improvement in clinical trials in ophthalmology. The percentage of eyes gaining three or more lines of vision in the first year of the study was significantly greater with aflibercept than the other two agents: Sixty-seven percent versus 41 percent for bevacizumab versus 50 percent for ranibizumab. But as we have seen previously, these differences disappeared in the second year, with 58 percent, 52 percent and 55 percent of eyes gaining three lines of vision respectively at the two-year final end point. These findings call into question the clinical importance of the differences between the three agents seen at one year.
There were important differences seen in the effectiveness of the three agents in reducing DME as measured by OCT CST. In the eyes with better baseline vision, bevacizumab reduced edema about 50 percent less than the other two agents across the entire two years of the study (Figure 3). In eyes with worse baseline vision, bevacizumab was less effective than the other two agents in the first year, but in year two caught up with ranibizumab but not aflibercept (Figure 4). Additionally, eyes treated with bevacizumab were half as likely to achieve a normal OCT CST of <250 µm at one year in eyes with better baseline vision. In eyes with worse baseline vision, only 46 percent of bevacizumab eyes versus 66 percent of ranibizumab and 75 percent of aflibercept eyes achieved this degree of anatomic improvement.
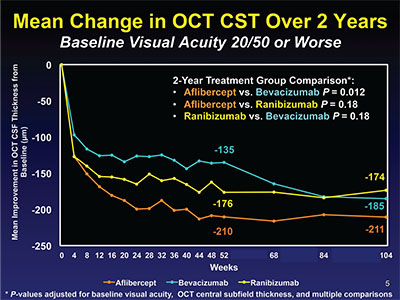 |
| Figure 4. In Protocol T, year one, the bevacizumab group was inferior to the other two groups, but caught up in year two. |
In summary, Protocol T demonstrated that all three anti-VEGF agents are highly effective treatments for center-involving DME, with significant improvements in vision and reductions in macular edema on OCT at one year that were sustained through two years with fewer injections and lasers than in the first year. In eyes with better baseline vision of 20/32 to 20/40, vision gains of about eight letters were seen in all three groups at one year and maintained at two years. But in eyes with 20/50 or worse baseline vision, aflibercept was superior to the other two agents at one year. In the second year, this superiority was diminished, however, with there being no statistically significant difference between aflibercept and ranibizumab, while bevacizumab remained inferior to aflibercept but not ranibizumab. More interesting is that the percentage of eyes gaining three lines of vision favored aflibercept at one year, but by year two there was no difference seen in the rates of three-line gainers, suggesting that in the long term there may be little clinical difference in the effectiveness of these three agents.
Some important questions remain unanswered. In eyes with better baseline vision, bevacizumab-treated eyes achieved 50 percent less reduction in DME on OCT, and were more likely to require laser. While this didn’t result in worse vision through two years, I’m concerned that persistent edema in this group might result in worse vision in the long term unless alternative treatments are given. Because it’s more effective at reducing edema and may be more effective at improving vision in eyes with good baseline vision and thicker maculae than bevacizumab,12 and also is less expensive than aflibercept, I prefer ranibizumab for this subgroup. Although in the worse baseline vision subgroup ranibizumab caught up to aflibercept by two years, I believe that the more rapid improvement seen with aflibercept in the first year cannot be ignored; I still prefer initiating treatment with that agent in this subgroup.
Finally, it’s important to realize that these results give no information on the effectiveness of switching agents when there is persistent edema. As stated earlier, this protocol tolerates persistent edema, but I acknowledge that in clinical practice most retinal specialists won’t tolerate persistent swelling and will either switch to a different anti-VEGF agent, add laser or use steroids when there is persistent edema after three to six injections. There are currently no clinical trials to confirm the effectiveness or necessity of switching agents. The treatment protocol used in this study is a useful one that gives excellent results, and can be followed with that knowledge. REVIEW
Dr. Wells is in private practice at Palmetto Retina Center and is chairman of the Department of Ophthalmology of the Palmetto Health-USC Medical Group in Columbia. He can be reached at jackwells@palmettoretina.com. He has received grant support from Genentech and Regeneron. The views expressed by Dr. Wells are his own and not the DRCR Network’s.
1. Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized trial. JAMA 2015;314:20:2137-2146.
2. Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372:13:1193–1203.
3. Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR, Jhaveri C, Melia M, Beck RW; Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016 Feb 27. pii: S0161-6420(16)00206-2. doi: 10.1016/j.ophtha.2016.02.022. [Epub ahead of print]
4. Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:6:1064-77.
5. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:4:789-801.
6. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014;121:11:2247-54.
7. The Diabetic Retinopathy Clinical Research Network. Rationale for the diabetic retinopathy clinical research network intravitreal anti-VEGF treatment and follow-up protocol for center-involved diabetic macular edema. Ophthalmology 2011;118:12:e5-e14.
8. Blankenship GW. Fifteen-year argon laser and xenon photocoagulation results of Bascom Palmer Eye Institute’s patients participating in the Diabetic Retinopathy Study. Ophthalmology 1991;98:2:125-128.
9. Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:6:1064-1077:e35.
10. Diabetic Retinopathy Clinical Research Network Writing Committee. Reproducibility of spectral-domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmol 2014;132:9:1113-22.
11. Thulliez M, Angoulvant D, Le Lez ML, et al. Cardiovascular events and bleeding risk associated with intravitreal antivascular endothelial growth factor antibodies: a systematic review and meta-analysis. JAMA Ophthalmol 2014;132:11:1317-26.
12. Wells JA, Glassman AR, Jampol LM, et al., for the Diabetic Retinopathy Clinical Research Network. Relationship of baseline visual acuity and retinal thickness on one-year efficacy of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema. JAMA Ophthalmol 2016;134:2:127-134.



