Abusive head trauma is a subset of non-accidental trauma that’s responsible for significant morbidity and mortality in infants and children. Ophthalmologists may be asked to examine children for signs of AHT, therefore it’s important to recognize ocular findings suggestive of physical abuse. Here, we’ll describe the signs and symptoms of AHT to look for when these children present.
Pathogenesis of Ocular Injuries in AHT
The incidence of AHT in infants less than 1 year old is approximately 30 per 100,000.1 Nearly 70 percent of survivors of AHT have long-term neurologic sequelae.2 The injuries are thought to be caused by rapid acceleration and deceleration caused by forceful shaking or direct head impact or both, with shearing forces at the vitreoretinal interface resulting in the typical intraocular manifestations.3 This damage is likely to occur in areas of strong attachment such as retinal vessels, vitreous base and the macula. The most common ocular manifestations of AHT are multi-layered intraocular hemorrhages extending to the retinal periphery, perimacular retinal folds and fibrosis, and traumatic retinoschisis.3-5 Typical extraocular injuries include diffuse unilateral or bilateral subdural hemorrhage, and diffuse brain injury, especially in the absence of significant external injuries.
Other contributing factors include hypoxia, anemia, reperfusion, autonomic vascular dysregulation, significant shifts in sodium balance, coagulopathy and elevated intracranial pressure.
Examination
Clinical findings in victims of abuse are variable, ranging from nonspecific ailments to acute life-threatening complications, such as severe respiratory distress, intracranial hypertension, loss of consciousness, seizure and shock. The classic triad of AHT is subdural hematoma, cerebral edema and retinal hemorrhage. Ophthalmologists should be aware of “red flags” that may indicate abuse, including poor nutrition, irritability, altered mental status, respiratory impairment, multiple fractures (especially in different stages of healing) and varying degrees of bruising.
A dilated fundus examination with indirect ophthalmoscopy should be performed to evaluate for intraocular signs of AHT. Additionally, a slit-lamp examination is helpful to identify signs of anterior segment trauma such as hyphema. This examination should preferably occur within 24 to 48 hours of initial presentation as intraretinal hemorrhages may resolve rapidly within days after the injury.6 However, it’s not possible to precisely determine the timing of injuries based on examination. In some patients where the pupil exam is needed for neurologic monitoring, it may be possible to dilate one eye at a time using a short-acting mydriatic agent to preserve the ability to monitor pupillary reactivity. If pharmacologic dilation is entirely contraindicated, it’s still advisable to attempt an undilated fundus examination rather than forgoing examination until pupillary dilation is permissible. Prior to pharmacologic dilation, perform an examination for a relative afferent pupillary defect to evaluate for possible optic neuropathy.
It’s important to document the exam findings in detail. When retinal hemorrhages are present, comment on the type (e.g., vitreous, preretinal, intraretinal, subretinal), number, size, location and distribution of the findings. Also note any additional findings, such as perimacular retinal folds or traumatic retinoschisis. In the absence of a clear external source of high-level accidental head trauma, severe findings should raise suspicion for AHT. Fundus photography is highly recommended to document findings.
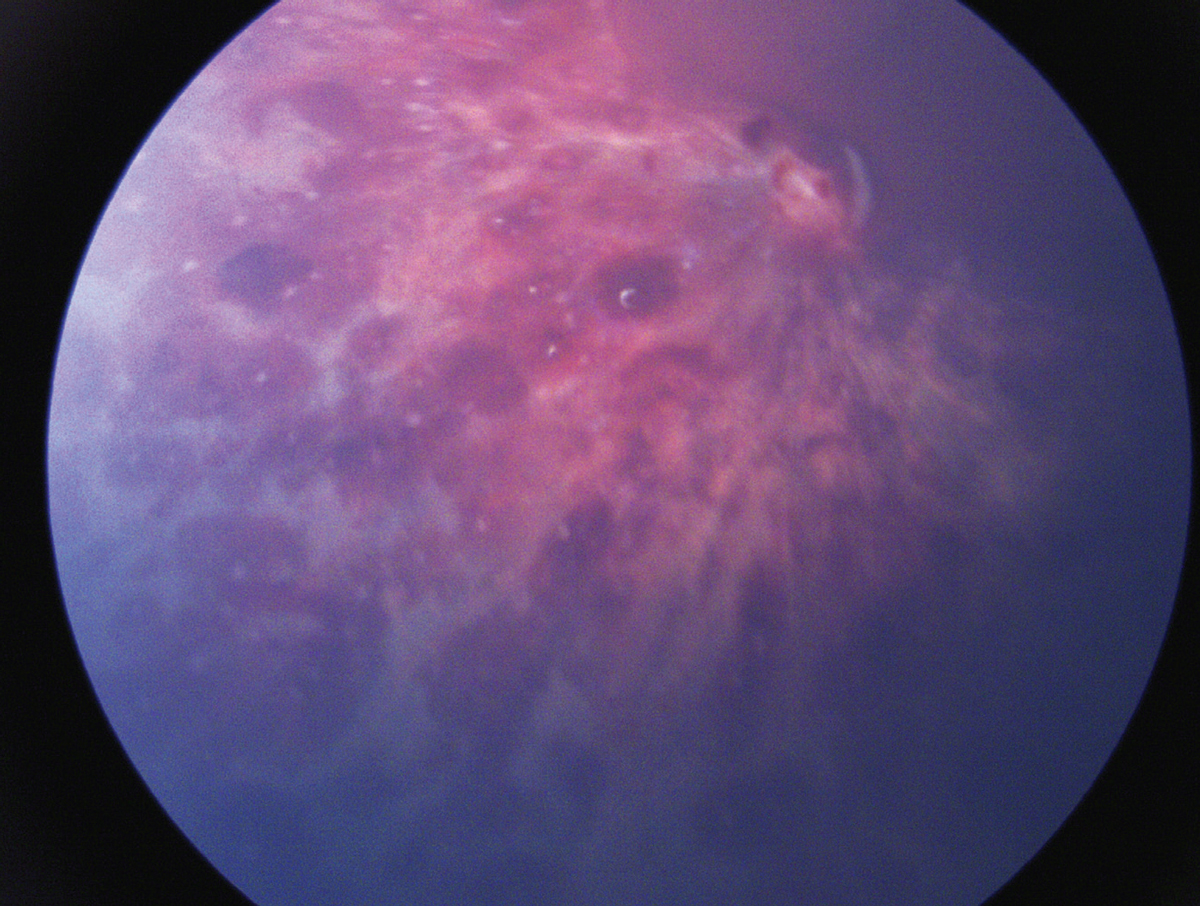 |
| Figure 1. Extensive multilayered retinal hemorrhages in a child with abusive head trauma. |
Intraocular Hemorrhages In AHT
The most common ocular abnormalities seen in AHT are intraocular hemorrhages, seen in around 75 percent of AHT patients (Figure 1). Therefore, intraocular hemorrhage remains the most reliable clinical feature of AHT. Intraocular hemorrhage has been found in children with accidental trauma, such as severe motor vehicle accidents, but hemorrhages from AHT typically occur in a pattern distinctly different from those associated with accidental trauma. Abusive head trauma-induced hemorrhages are typically found in all retinal layers, although they may be confined to the superficial layers (e.g., round or boat-shaped sub-internal limiting membrane hemorrhages, and splinter and flame-shaped nerve fiber and ganglion cell layer hemorrhages).3 Vitreous hemorrhage and choroidal or sub-retinal pigment epithelial hemorrhage may also be seen. The posterior pole is the most common location for retinal hemorrhages, though more than half of patients have hemorrhages extending to the mid-periphery and peripheral retina. Furthermore, nearly 75 percent of AHT patients with intraocular hemorrhage have bilateral findings.3
Intraretinal hemorrhages may resolve over the course of days, whereas preretinal hemorrhages may persist for weeks, thus resulting in rapid evolution of the examination findings, and necessitating examination within 24 for 48 hours of initial presentation in order to document the full extent of injuries.6 In cases of postmortem evaluation, histological examination may also support the clinical diagnosis of AHT by showing the presence of intraretinal hemorrhages, intrascleral hemorrhages, optic nerve sheath hemorrhages and perimacular folds.
Retina Research update: Study Identifies Uneven Treatment Patterns for DME Investigators set out to characterize long-term, real-world anti-VEGF treatment patterns in patients with diabetic macular edema, and found that while more than half discontinue intravitreal injections after about six months, one third of them restarted treatments after about 15 months. Theodore Leng, MD, MS, director of clinical and translational research, Byers Eye Institute at Stanford University School of Medicine, presented results from a retrospective analysis of 190,345 eyes of 147,687 patients in the IRIS Registry from 2015 through 2019.1 It’s the largest known follow-up to date of anti-VEGF treatment patterns for DME, he said. In any given year, about one-third of patients discontinued anti-VEGF treatments, he said, and 77 percent of eyes received only one anti-VEGF agent over an average follow-up of 2.3 years. Bevacizumab is the most commonly used agent, representing 53 percent of study eyes, followed by aflibercept (21 percent) and ranibizumab (11 percent). However, many patients switch agents, he noted. “As each year passed, bevacizumab use decreased by a mean 5.6 percent and on-label agent use increased by 6.5 percent,” he said. “Fifteen percent of eyes switched during the study period after 53 weeks, of which 74 percent switched from bevacizumab to an on-label agent.” Ten percent of eyes did the reverse, switching from an on-label agent to bevacizumab. The study also found that discontinuation rates were “mostly similar” regardless of baseline vision. “Although,” Dr. Leng added, “discontinuation with no re-initiation of injections during follow-up was highest in patients with vision of 20/100 or worse at baseline.” The study data were not robust enough to determine a difference between patients who discontinued treatment and those lost to follow-up for 12 months, Dr. Leng said. And comorbidities that may have influenced treatment patterns weren’t captured. “This is the largest and longest follow-up study known to date, extending out to six years, for evaluating patients with DME in the registry,” he said. “The reasons for switching agents should be further explored.” Roche funded the study. Dr. Leng is a consultant to Genentech/Roche and Regeneron. 1. Leng T, Garmo V, Tabano D, et al. Long-term real-world treatment patterns among patients with diabetic macular edema initiating anti-VEGF: 6-year follow-up using the IRIS Registry. Paper presented at the American Society of Retina Specialists annual meeting; July 15, 2022; New York, NY. |
Retinal Folds and Traumatic Retinoschisis
Perimacular or paramacular retinal folds and traumatic retinoschisis are less common than retinal hemorrhages, occurring in approximately 10 percent of AHT cases.3 As described above, the pathogenesis is thought to be due to intense shearing forces at the vitreoretinal interface. Although the presence of retinal folds or traumatic retinoschisis is not diagnostic of AHT, there are only rare case reports of these findings occurring in patients with alternate mechanisms of head trauma, including fatal motor vehicle accidents and fatal crush injuries.7-9 It can be challenging to distinguish traumatic retinoschisis from preretinal hemorrhages, as traumatic retinoschisis may sometimes involve only superficial retinal structures (e.g., internal limiting membrane and/or retinal nerve fiber layer), and may be accompanied by hemorrhage into the schisis cavity (Figure 2A). Additionally, there may be overlying vitreous or preretinal hemorrhage obscuring the view. On binocular indirect ophthalmoscopy, traumatic retinoschisis may be identified as an elevation of the internal limiting membrane or other retinal layers that may be bordered by retinal folds or circumlinear hypopigmented lines.10 Traumatic schisis cavities often don’t resolve (Figure 2B), and may be accompanied by pre-retinal or subretinal fibrosis, pigmentary changes or macular holes (Figure 3).
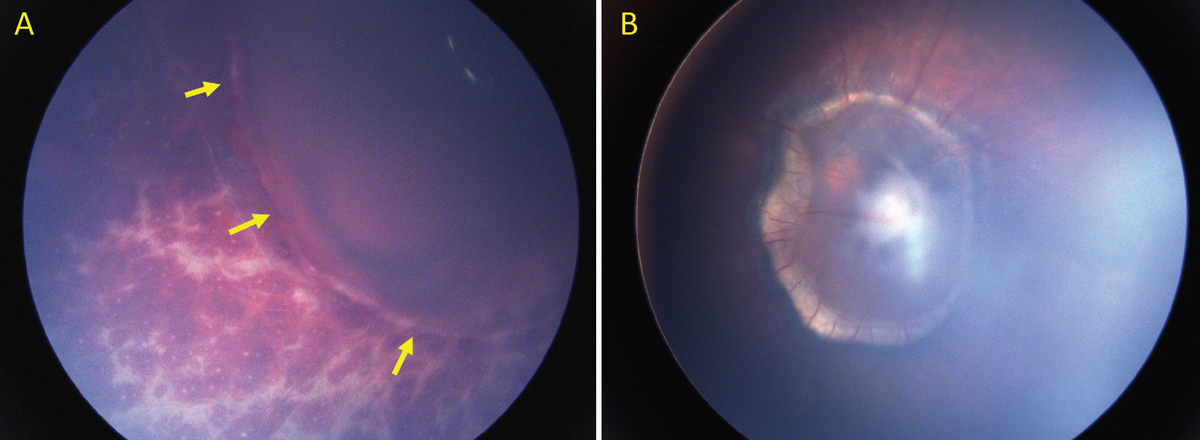 |
| Figure 2. Traumatic retinoschisis in a child with AHT. (A) Acute presentation of AHT with multilayered retinal hemorrhages and traumatic retinoschisis (arrows). Hemorrhage is seen within the schisis cavity. (B) Two years after the injury, the schisis persists and is accompanied by pre-retinal fibrosis and a ring of subretinal fibrosis. |
Differential Diagnosis
It’s important to consider relevant history and physical examination findings, as well as radiologic and laboratory studies, when evaluating the likelihood of AHT. Furthermore, it’s possible for more than one etiology to contribute to clinical findings, thus the confirmation of an alternate diagnosis shouldn’t exclude the possibility of concomitant abusive head trauma.
• Accidental head injury. Victims of AHT often have limited visible external injuries, whereas in case reports of accidental head injuries resulting in retinal manifestations similar to those seen in AHT there is typically significant external injury. Moreover, in cases of accidental head injury with retinal findings similar to those seen in AHT, the injury is often severe and commonly fatal (e.g., high speed motor vehicle accident or crush injury to head).
• Birth trauma. Birth-related retinal hemorrhages are common and may be seen in around one-third of newborns and typically resolve within the first month of life. Although there are many clinical features that may be shared between the two entities, birth-related retinal hemorrhages are unlikely to involve vitreous, preretinal, or subretinal hemorrhages, and are unlikely to persist beyond 1 month of age.11
• Hematologic abnormalities. It’s important to check laboratory tests to evaluate for hematologic abnormalities in patients with suspected AHT in the absence of other evidence of physical abuse. It’s possible for hematologic abnormalities such as coagulopathy (including hemophilia, thrombocytopenia and vitamin K deficiency) or hematologic malignancy to result in intracranial hemorrhage and, less commonly, retinal hemorrhages. Retinal hemorrhages due to hematologic abnormalities are usually less severe than in cases of AHT, and are often confined to the posterior pole. In patients with leukemia, it’s possible for the retinal hemorrhages to be more severe, more diffuse and accompanied by white-centered hemorrhages. Hematologic abnormalities aren’t associated with retinal folds or retinoschisis, and the presence of those findings should increase suspicion for abusive head trauma.12
• Intracranial hemorrhage (Terson syndrome). Intraocular hemorrhage may be associated with intracranial hemorrhage due to vascular abnormality or trauma. Various mechanisms have been proposed to explain the findings, including intracranial blood passing through the optic nerve sheath, or alternatively, acute increase in pressure transmitted through the optic nerve sheath resulting in compression of the central retinal vessels and subsequent microvascular rupture.13,14 The most common manifestations are vitreous or preretinal hemorrhages, whereas widespread multi-layered or intraretinal hemorrhages as seen in abusive head trauma aren’t common.
• Papilledema. In cases of optic disc edema due to sustained elevation of intracranial pressure, it’s possible to see superficial peripapillary hemorrhages (e.g., flame-shaped hemorrhages). The presence of widespread and/or multi-layered retinal hemorrhages isn’t consistent with papilledema alone and you should consider alternate or additional diagnoses.
• Purtscher retinopathy or resuscitation trauma. Purtscher retinopathy may occur in cases of acute severe thoracic compression. It manifests as white retinal lesions (Purtscherflecken) that are caused by infarcts of the retinal nerve fiber layer similar to cotton wool spots, and can be associated with retinal hemorrhages and peripapillary retinal edema. Thoracic trauma including trauma inflicted by chest compressions performed during cardiopulmonary resuscitation don’t cause multi-layered retinal hemorrhages such as those seen in cases of abusive head trauma.
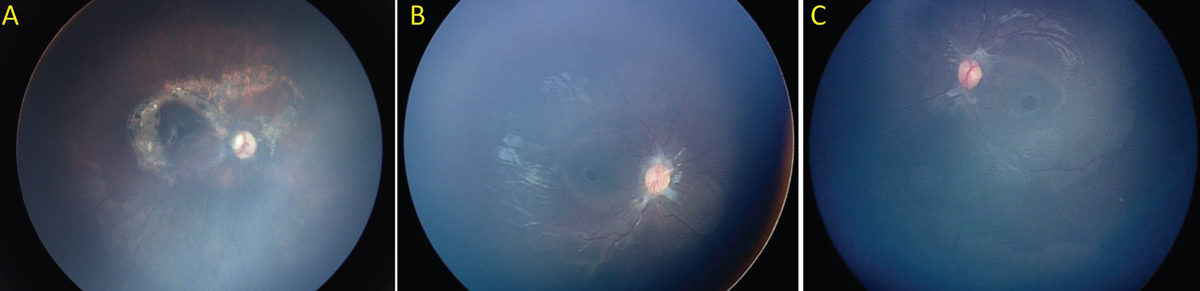 |
| Figure 3. Chronic complications of abusive head trauma. (A) Perimacular subretinal fibrosis and pigmentary changes in a 3-year-old child with a history of AHT. (B-C) Pre-retinal fibrosis and bilateral full thickness macular holes in a 4-year-old child with a history of abusive head trauma. |
Prognosis and Management
The overall prognosis for victims of abusive head trauma is poor, with a reported mortality rate of around 25 percent.15
Ocular findings shown to be independent risk factors for increased mortality include poor visual acuity at initial presentation, diminished pupillary response, optic disc edema and diffuse retinal hemorrhages or retinal folds.16–18 Most survivors of abusive head trauma have long-term neurologic sequelae. Visual prognosis is guarded, and most survivors suffer permanent vision loss in one or both eyes due to brain and/or ocular injuries.2,19,20 Ocular etiologies of permanent vision loss from abusive head trauma include retinal detachment, macular scarring or fibrosis, and amblyopia associated with non-clearing vitreous hemorrhage. Regular follow-up with an ophthalmologist is needed to maximize visual outcome.
In conclusion, abusive head trauma is a significant cause of morbidity and mortality in infants and children. Diagnosis of AHT requires accurate clinical history, a dilated fundus examination and neuroimaging. Examination by an ophthalmologist is an important part of the evaluation for suspected AHT, as funduscopic examination can reveal findings that have high specificity for AHT. The funduscopic examination should take place within 24 to 48 hours of presentation. Even if pharmacologic pupillary dilation is contraindicated, you should attempt an undilated exam rather than deferring examination. Multiple, bilateral and multi-layered retinal hemorrhages that extend to the periphery of the retina, as well as retinal folds and traumatic retinoschisis are findings that are highly specific for AHT. When possible, perform fundus photography to document your exam findings. If you identify findings suspicious for AHT, notify appropriate child protective services personnel, and arrange for a comprehensive evaluation for additional signs of abuse. Prognosis is guarded, unfortunately, due to a high mortality rate and high incidence of long-term visual and neurologic sequelae in survivors. The patient needs to have follow-up ophthalmologic exams to address any reversible causes of vision loss and maximize visual potential.
Dr. Regillo is the director of the Retina Service of Wills Eye Hospital, a professor of ophthalmology at Thomas Jefferson University School of Medicine and the principle investigator for numerous major international clinical trials.
Dr. Yonekawa is an assistant professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University. He serves on the Education Committee of the American Society of Retina Specialists and on the Executive Committee for the Vit Buckle Society, where he is also the vice president for academic programming.
Dr. Koretz Koretz is a first-year vitreoretinal surgery fellow at the Shiley Eye Institute at the University of California, San Diego.
Dr. Song is a senior vitreoretinal fellow at the Shiley Eye Institute.
Dr. Nudleman is an associate professor of clinical ophthalmology, the Viterbi Family Chair for Retinal Vascular Diseases and co-director of the Retina Division at the Shiley Eye Institute.
None of the authors has any financial interest in any of the products mentioned in the article.
1. Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH. A population-based study of inflicted traumatic brain injury in young children. JAMA 2003;290:5:621-626.
2. Barlow KM, Thomson E, Johnson D, Minns RA. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics 2005;116:2:e174-185.
3. Bhardwaj G, Chowdhury V, Jacobs MB, Moran KT, Martin FJ, Coroneo MT. A systematic review of the diagnostic accuracy of ocular signs in pediatric abusive head trauma. Ophthalmology 2010;117:5:983-992.e17.
4. Levin AV. Ophthalmology of shaken baby syndrome. Neurosurg Clin N Am 2002;13:2:201-211.
5. Sturm V, Landau K, Menke MN. Optical coherence tomography findings in shaken baby syndrome. American Journal of Ophthalmology 2008;146:3:363-368.
6. Binenbaum G, Chen W, Huang J, Ying G shuang, Forbes BJ. The natural history of retinal hemorrhage in pediatric head trauma. J AAPOS 2016;20:2:131-135.
7. Lueder GT, Turner JW, Paschall R. Perimacular retinal folds simulating nonaccidental injury in an infant. Archives of Ophthalmology 2006;124:12:1782-1783.
8. Lantz PE, Sinal SH, Stanton CA, Weaver RG. Perimacular retinal folds from childhood head trauma. BMJ. 2004;328:7442:754-756.
9. Kivlin JD, Currie ML, Greenbaum VJ, Simons KB, Jentzen J. Retinal hemorrhages in children following fatal motor vehicle crashes: A case series. Archives of Ophthalmology 2008;126:6:800-804.
10. Shouldice M, Al-Khattabi F, Thau A, McIntyre S, Ng WKY, Levin AV. Traumatic macular retinoschisis in infants and children. Journal of American Association for Pediatric Ophthalmology and Strabismus 2018;22:6:433-437.
11. Emerson MV, Pieramici DJ, Stoessel KM, Berreen JP, Gariano RF. Incidence and rate of disappearance of retinal hemorrhage in newborns. Ophthalmology 2001;108:1:36-39.
12. Thau A, Saffren B, Zakrzewski H, Anderst JD, Carpenter SL, Levin A. Retinal hemorrhage and bleeding disorders in children: A review. Child Abuse & Neglect 2021;112.
13. Ogawa T, Kitaoka T, Dake Y, Amemiya T. Terson syndrome: A case report suggesting the mechanism of vitreous hemorrhage. Ophthalmology 2001;108:9:1654-1656.
14. Muller PJ, Deck JHN. Intraocular and optic nerve sheath hemorrhage in cases of sudden intracranial hypertension. Journal of Neurosurgery 1974;41:2:160-166.
15. Ferguson NM, Sarnaik A, Miles D, Shafi N, Peters MJ, Truemper E, Vavilala MS, Bell MJ, Wisniewski SR, Luther JF, Hartman AL, Kochanek PM. Abusive head trauma and mortality – An analysis from an international comparative effectiveness study of children with severe traumatic brain injury. Crit Care Med 2017;45:8:1398-1407.
16. Mills M. Funduscopic lesions associated with mortality in shaken baby syndrome. J AAPOS 1998;2:2:67-71.
17. McCabe CF, Donahue SP. Prognostic indicators for vision and mortality in shaken baby syndrome. Arch Ophthalmol 2000;118:3:373-377.
18. Wilkinson WS, Han DP, Rappley MD, Owings CL. Retinal hemorrhage predicts neurologic injury in the shaken baby syndrome. Arch Ophthalmol 1989;107:10:1472-1474.
19. Kivlin JD, Simons KB, Lazoritz S, Ruttum MS. Shaken baby syndrome. Ophthalmology 2000;107:7:1246-1254.
20. Matthews GP, Das A. Dense vitreous hemorrhages predict poor visual and neurological prognosis in infants with shaken baby syndrome. Journal of Pediatric Ophthalmology & Strabismus 1996;33:4:260-5.



