Retinal vein occlusions are a common but heterogenous group of retinal disorders characterized by impaired venous return of the retinal circulation. Though RVOs share common clinical features, they’re distinct entities with their own risk factors, prognosis and—sometimes—treatments. There’s also a wide spectrum of clinical severity of RVO and, if left untreated, RVO can lead to permanent vision impairment. Early recognition and prompt treatment, if needed, are key to preserving vision. Here, I’ll outline the features of the different kinds of vein occlusions, the best way to diagnose them and the most effective course of treatment.
Classifying Occlusions
Classification of RVO can be divided into branch retinal vein occlusion, hemiretinal vein occlusion and central retinal vein occlusion, depending on the location of the obstruction. If the occlusion occurs within or posterior to the optic nerve head (often a thrombus in the central retinal vein near the lamina cribrosa), it’s termed a CRVO. If the occlusion is at the major bifurcation of the central retinal vein, it’s an HRVO, and any obstruction within a tributary vein (often a thrombus at the arteriovenous crossing point) is a BRVO. Often, HRVO is a considered a separate condition that can behave in a way that’s between a BRVO and CRVO.1,2
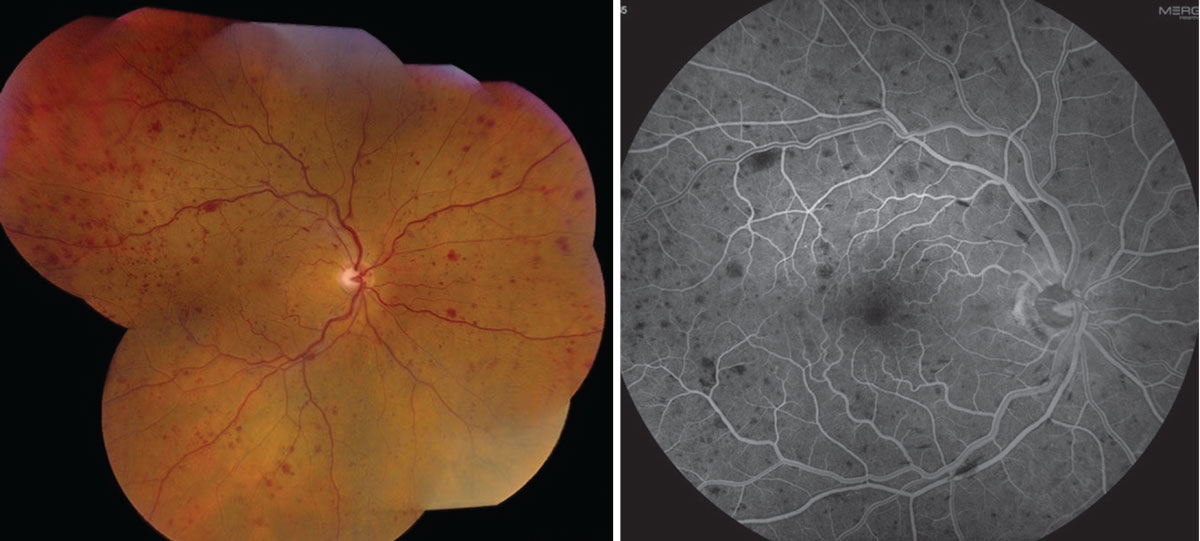 |
| Figure 1. Fundus photograph and fluorescein angiogram of a non-ischemic central retinal vein occlusion. |
Epidemiology and Risk Factors
Together, RVOs represent the second leading cause of retinal vascular blindness after diabetic retinopathy. BRVO is more common than CRVO.3-6 Worldwide prevalence of BRVO is estimated to be 0.4 percent, and CRVO around 0.08 percent, with a symmetrical distribution between men and women and increased risk with older age.7 In the Beaver Dam Eye Study, at 15 years the cumulative incidence of BRVO was 1.8 percent, vs. 0.5 percent for CRVO.8 The Blue Mountain Eye Study showed a 0.7-percent incidence in patients younger than 60 years, increasing to 4.6 percent in patients 80 years and older.5
The greatest predictor of developing RVO is an RVO in the contralateral eye. Individuals with BRVO in one eye have a 10-percent risk of any RVO in the contralateral eye within three years.9 The estimated risk of contralateral involvement in a patient with CRVO is approximately 1 percent per year, but increases to 7 percent at five years.9,10
RVOs have been associated with certain systemic vascular risk factors, including hypertension, hyperlipidemia, diabetes, active smoking and peripheral vascular disease.3-5,11,12 Of these systemic risk factors, one meta-analysis found that 47.9 percent of RVO cases were attributed to hypertension, 20.1 percent to hyperlipidemia and 4.9 percent to diabetes.13 The study concluded that hypertension and hyperlipidemia were common risk factors for all forms of RVO in adults, whereas diabetes was less significant due to its inconsistent association with BRVO. Some studies have shown an increased risk of cerebrovascular and cardiovascular disease in patients with RVO, including a greater risk of developing acute myocardial infarction after a diagnosis of RVO, although other studies have shown similar rates of stroke and myocardial infarction.14-17
There is some controversy surrounding hypercoagulable states and RVO. One meta-analysis of 26 studies found that thrombophilic risk factors, hyperhomocysteinemia and anticardiolipin antibodies were significantly independently associated with RVO.18 Other associations include proteins C and S deficiency, high alpha-2 globulin concentrations, higher activated factor VII concentrations, oral contraception use and increased blood viscosity.12,19
Additionally, open-angle glaucoma is a common ocular comorbidity in RVO patients.11,20 Glaucoma, along with sleep apnea, is more common in CRVO than BRVO.21,22
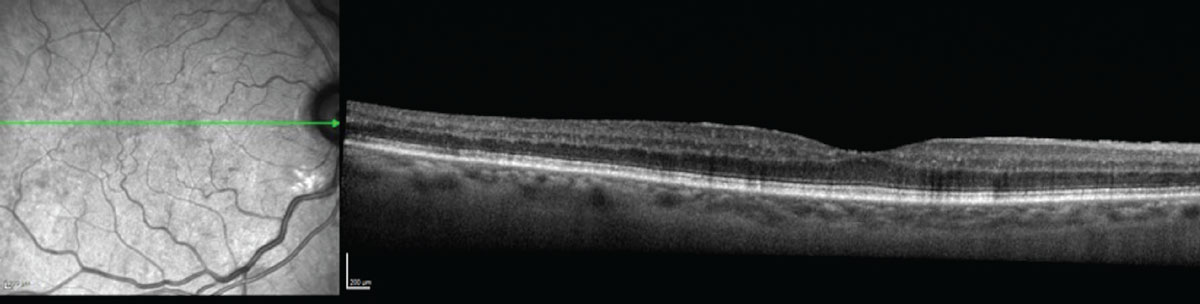 |
| Figure 2. Optical coherence topography of the patient from Figure 1 didn’t show any significant macular edema. |
Clinical Features
RVO patients are at risk of vision loss from several complications of the interrupted blood flow, including macular edema, macular ischemia, optic neuropathy, vitreous hemorrhage and tractional retinal detachment. However, symptoms related to an RVO can be subtle, especially if the severity is mild or the impacted area is outside the macula. Acute RVO commonly presents with painless visual disturbances. Visual field abnormalities can be present but are rarely symptomatic.23 Increased intravenous pressure can result in vascular tortuosity, retinal hemorrhages (superficial flame-shaped and deep blot), cotton wool spots and optic nerve edema. RVO can be classified by anatomic location. BRVO occurs in one retinal quadrant and can be distinguished by hemorrhage in that area. HRVO patients show findings only in the impacted hemifield, while CRVO patients can show retinal hemorrhages in all four quadrants. Congestion of the capillary bed can lead to macular edema, metamorphopsia and decreased visual acuity. Severe congestion can also result in vitreous hemorrhage, which can be difficult to distinguish from vitreous hemorrhage related to ocular neovascularization.
BRVO typically occurs at arteriovenous crossings where the artery and vein share an adventitial sheath. The artery has been observed to cross the vein anteriorly in 98 to 99 percent of BRVO, compared with approximately 60 to 70 percent of unaffected arteriovenous crossings.4,24,25 This has been hypothesized to occur due to thickening of the overlying artery, which causes narrowing of the vein, with subsequent vascular turbulence and endothelial damage contributing to venous thrombosis. The superotemporal quadrant is most commonly involved (58.1 percent of eyes), followed by the inferotemporal quadrant (29 percent) and outside of the temporal quadrants (12.9 percent).3,25
Eyes with more nonperfusion carry a greater risk of ocular neovascularization and a guarded visual prognosis.26 CRVO generally causes greater degrees of vision loss and carries a more guarded prognosis. Abnormal new blood vessel growth can invade the iris, angle, optic nerve and retina. If the angle is involved, neovascular glaucoma can result. IOP elevation can occur within one month of onset or later, leading to the term “90-day glaucoma.”27
With time, collateralization (retina-retina and retina-choroid anastomoses) can bypass the obstruction and improve clinical signs, and the hemorrhages, cotton wool spots and optic nerve edema can improve.
Imaging
While RVO is a clinical diagnosis, supplemental imaging can help confirm the diagnosis, monitor response to treatment and reveal complications such as macular edema and neovascularization.
Fluorescein angiography can illustrate the characteristic finding of delayed filling of the occluded retinal vein. In chronic cases, FA may only reveal microvascular changes, including microaneurysms and telangiectatic collateral vessels, after retinal hemorrhages have resolved. FA also permits visualization of peripheral capillary nonperfusion and macular ischemia, and detection of subtle neovascularization that may not be clinically apparent. Historically, FA was also used to classify RVO into groups of perfused, nonperfused or indeterminate by evaluating for five or more disc areas of capillary nonperfusion in the Branch Vein Occlusion Study (BVOS), and ten or more disc areas in the Central Vein Occlusion Studies (CVOS).26,28 According to the CVOS, CRVO were classified as ischemic if FA revealed more than 10 disc diameters of retinal capillary nonperfusion; they’re considered perfused if fewer than 10 disc diameters of ischemia are present, or as indeterminate if accurate determination of the degree of nonperfusion can’t be estimated due to significant retinal hemorrhage.29 While this framework was useful for study purposes, it’s been largely outdated with ultra-widefield imaging and its ability to help us easily detect nonperfusion and neovascularization.
Optical coherence tomography is critical in confirming the presence of macular edema, including cystoid changes and subretinal fluid, and monitoring response to treatment. Chronic cases can show ellipsoid zone loss from longstanding edema or ischemia. Images obtained with OCT can also provide additional information such as vitreoretinal interface abnormalities, neurosensory detachments and/or loss of outer retina integrity that may further limit vision or guide therapy. OCT angiography can also be helpful in diagnosing occult cases. OCTA allows imaging of the perfused retinal vasculature by acquiring high speed, sequential OCT A-scans at the same retinal locus and then processing complex digital subtraction algorithms to analyze differences created by the moving columns of blood. This technology can show a reduction of blood vessel density, mainly of the deep retinal plexus, in RVO.
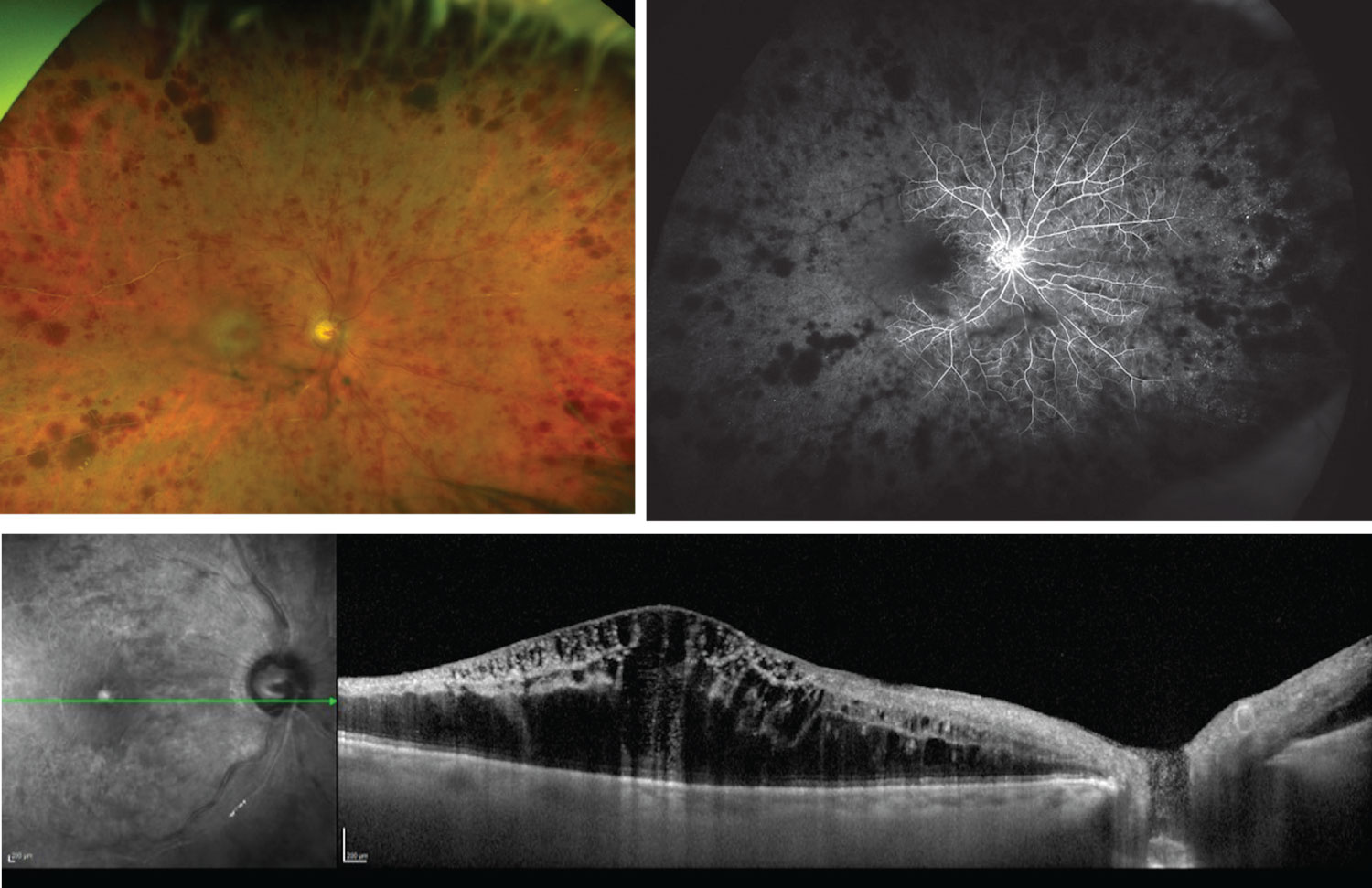 |
| Figure 3. The patient from Figure 1, three months later. An ischemic CRVO has developed, as seen on the fundus photograph and fluorescein angiogram (top), with edema visible on OCT (bottom). |
Treatment
Unfortunately, no treatment can reverse RVO, though attempts have been made to create anastomoses through surgery and laser, to relieve the obstruction through thrombolytic administration and bypass the congestion via optic nerve sheathotomy.30-33 As a result, the goal is to manage complications of macular edema and neovascularization.
Here, we’ll discuss several landmark trials that help provide guidance in improving visual outcomes for both BRVO and CRVO. We’ll also discuss the evolution of our treatment strategies, working our way from initial therapies that were used to our most current approaches.
• Laser for macular edema. The most common visually threatening complication of RVO is macular edema. In 1986, the National Eye Institute led the BVOS to examine laser treatment for ME from BRVO. In the study, researchers randomized patients with perfused BRVO and visual acuity of 20/40 or worse with ME to grid laser or observation. More patients gained two lines or more of visual acuity from baseline with laser than those without treatment (65 percent vs. 37 percent). Additionally, patients with laser were almost twice as likely to have a final visual acuity greater than 20/40. Given these findings, macular grid laser became the standard of care for ME associated with BRVO.28 Interestingly, the CVOS demonstrated a lack of benefit with respect to the use of macular grid laser for ME in CRVO. While grid laser photocoagulation was historically the gold-standard treatment for BRVO, intravitreal pharmacotherapy has largely replaced laser as the intervention of choice for both BRVO and CRVO.
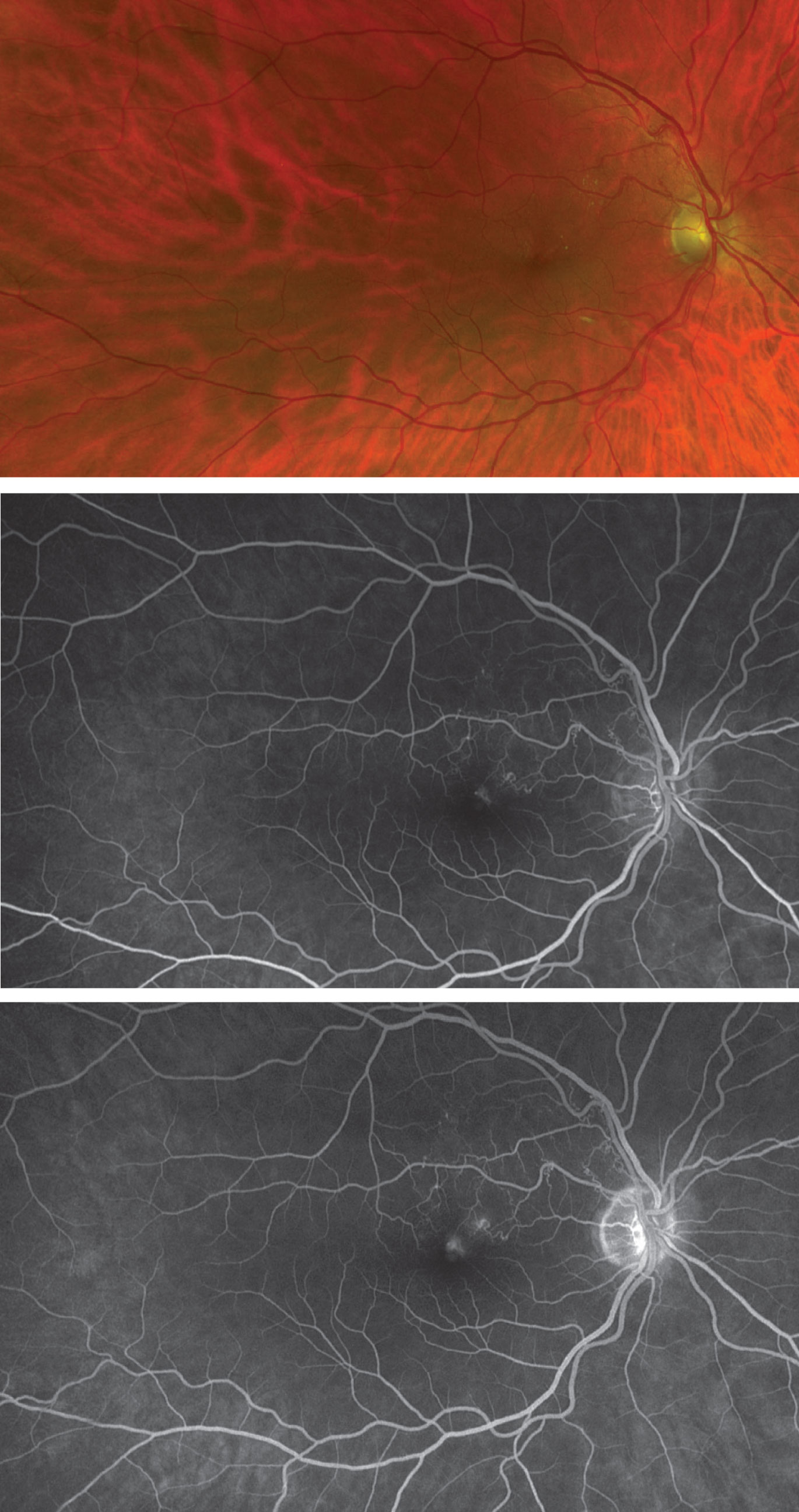 |
| Figure 4. Fundus photograph and early (middle) and late (bottom) fluorescein angiograms showing twig branch retinal vein occlusion. |
More recently, researchers have explored the use of peripheral “targeted” laser photocoagulation on angiographically nonperfused retina to decrease the burden of treatment associated with intravitreal anti-vascular endothelial growth factor injections. However, no study has demonstrated a benefit.34-36
• Intravitreal steroids for macular edema. Intravitreal corticosteroids are an effective treatment for ME secondary to RVO.37-41 Use of intravitreal triamcinolone injection in the 2009 SCORE study resulted in superior visual outcomes in patients with CRVO compared to observation, but not compared to grid photocoagulation.37,38
The dexamethasone intravitreal implant 0.7 mg (Ozurdex; Allergan), in the GENEVA trial and in a head-to-head comparison versus ranibizumab in the COMRADE B and C trials, led to rapid visual acuity gain for two months (comparable to ranibizumab). However, visual acuity gain wasn’t sustained after month three in any of the trials, resulting in inferior overall performance compared to ranibizumab from month three to six.39-41 These outcomes may be related to undertreatment in the dexamethasone arm compared to anti-VEGF therapy, however.39-41 Also, it’s well-known that intravitreal corticosteroids can be associated with ocular hypertension and cataract progression. Even so, some studies have shown that intravitreal steroids may be useful for the treatment of ME unresponsive to anti-VEGF therapy.42,43
• Intravitreal anti-VEGF therapy. Intravitreal injection of anti-VEGF agents has become first-line therapy for ME secondary to RVO since numerous prospective studies have revealed its remarkable therapeutic effects.1,35,44–54 More than half of patients with nonischemic RVO will achieve rapid improvement in visual acuity and reduction in retinal thickness shortly after initiation of anti-VEGF therapy, and these improvements are largely maintained with adequate retreatment.1,6-19,35,44-54 Early initiation (less than three months from onset) of anti-VEGF therapy appears to lead to the greatest improvement in visual acuity.1,35,44-54
There don’t seem to be definitive differences in efficacy and safety among the different anti-VEGF agents.1,55 Different injection frequency practices have been evaluated, however. The SHORE study demonstrated that an as-needed regimen with monthly follow-up, after seven monthly injections, was as effective as a monthly treatment approach.49 Many of the pivotal trials have mandated a loading period, but other studies have shown that one or two injections may be enough before switching to PRN in cases where there has been complete ME resolution.56 During initial therapy, follow-up intervals beyond two months aren’t recommended. Visual acuity was maintained with bimonthly monitoring in the CRYSTAL study but not with quarterly monitoring in COPERNICUS.50,54
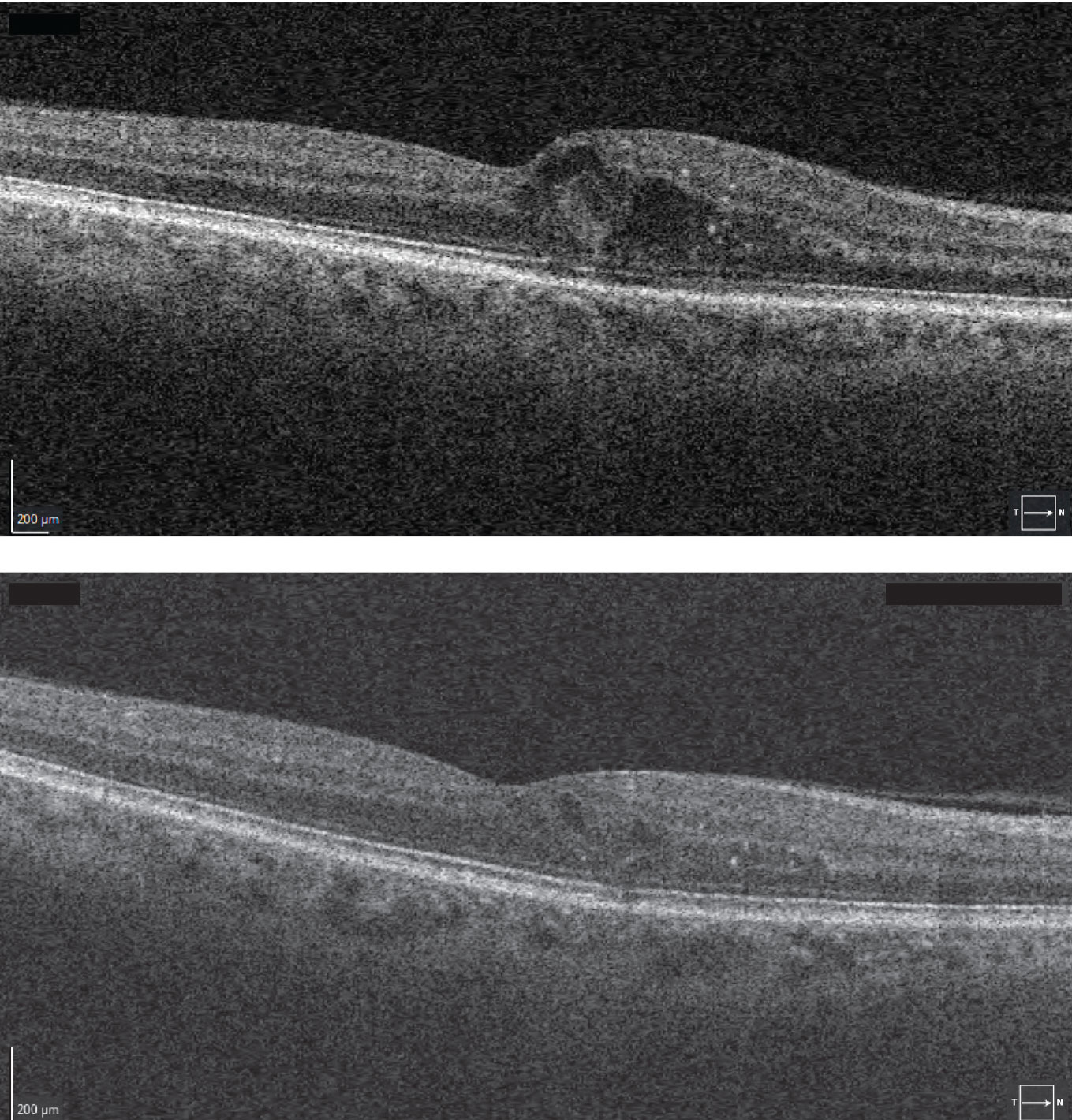 |
|
Figure 5. The macular edema of the patient in Figure 4 initially seen on optical coherence tomography (top) significantly improved on OCT one month after anti-VEGF treatment (bottom). |
Other studies suggest that switching anti-VEGF agents, or switching to a steroid agent, should be considered in eyes that don’t show a complete anatomic response.57 Switching anti-VEGF agents, particularly to aflibercept (Eylea, Regeneron), may be beneficial for extending treatment intervals as well. In NEWTON and other studies, refractory ME unresponsive to ranibizumab (Lucentis, Genentech) or bevacizumab (Avastin, Genentech) was anatomically improved with aflibercept, and treatment intervals were able to be extended.58-60
Ongoing Studies
New therapeutics continue to be tested for macular edema. Two agents were recently tested in Phase III studies but, unfortunately, both studies were stopped.
Brolucizumab (Beovu, Novartis), an anti-VEGF injection approved for neovascular age-related macular degeneration, was being investigated in the Phase III RAPTOR and RAVEN studies for RVO; it included four-week dosing intervals. However, both studies were stopped, given safety concerns after higher rates of intraocular inflammation were seen in the brolucizumab group of another clinical trial with four-week dosing.61 Additionally, the Phase III SAPPHIRE study examined suprachoroidal triamcinolone acetonide (Clearside) in conjunction with aflibercept for RVO but the combination didn’t meet its primary endpoint compared to aflibercept alone so the study was stopped. Both of these agents showed promise, given their new mechanisms of action and modes of delivery but, unfortunately, they weren’t able to continue.62
Two agents are currently being investigated in Phase III studies for RVO. Faricimab (Genentech), a bispecific antibody targeting vascular endothelial growth factor-a and angiopoietin-2 is being investigated in the COMINO and BALATON RVO trials in comparison to aflibercept.63 Additionally, KSI-301 (Kodiak Sciences), an anti-VEGF injection, is being compared to aflibercept in the Phase III BEACON study.64
Ocular Neovascularization
Besides macular edema, the other major visually threatening RVO complication is ocular neovascularization. Hypoxia and capillary nonperfusion can upregulate inflammatory cytokines, including VEGF, which promote increased vascular permeability and angiogenesis. The CVOS studied the risk of ocular neovascularization with and without pre-emptive panretinal photocoagulation, as determined by initial perfusion status. The study found that ocular neovascularization developed in 35 percent of ischemic or indeterminate eyes, compared with 10 percent of nonischemic eyes.26 Preemptive PRP reduced the likelihood of ocular neovascularization, but prompt resolution of ocular neovascularization occurred more frequently when laser treatment was deferred.26 Given these findings, the CVOS group recommended deferring PRP until ocular neovascularization develops.
Neovascular glaucoma has a guarded prognosis and treatment with PRP can be challenging if the patient is in pain, if there’s significant corneal edema or if there’s vitreous hemorrhage. Anti-VEGF medications can be used temporarily to treat neovascularization until PRP laser can be applied.
In conclusion, recognizing the clinical features of RVO and promptly treating the complications of macular edema and neovascularization is important to obtaining the best possible clinical outcomes. Unfortunately, there’s no direct treatment for improving perfusion. Instead, our current treatment focuses on minimizing and managing the complications of macular edema and neovascularization. Good treatments exist, including anti-VEGF therapy, intravitreal corticosteroids and panretinal photocoagulation. Future directions for therapy include novel, longer-acting anti-VEGF agents and new drug delivery systems.
Dr. Talcott is an assistant professor of ophthalmology and the residency associate program director at the Cleveland Clinic’s Cole Eye Institute. She is a consultant to Genentech and has received grants from Zeiss and Regenxbio. Address correspondence and requests for reprints to: Katherine E. Talcott, MD; phone: (440) 788-4040; e-mail: talcotk@ccf.org.
Dr. Regillo is the director of the Retina Service of Wills Eye Hospital, a professor of ophthalmology at Thomas Jefferson University School of Medicine and the principle investigator for numerous major international clinical trials.
Dr. Yonekawa is an assistant professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University. He serves on the Education Committee of the American Society of Retina Specialists and on the Executive Committee for the Vit Buckle Society, where he is also the vice president for academic programming.
1. Scott IU, VanVeldhuisen PC, Ip MS, et al. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: The SCORE2 randomized clinical trial. JAMA 2017;317:20:2072.
2. Scott IU, VanVeldhuisen PC, Oden NL, et al. Baseline characteristics and response to treatment of participants with hemiretinal compared with branch retinal or central retinal vein occlusion in the Standard Care vs COrticosteroid for REtinal Vein Occlusion (SCORE) study: SCORE Study Report 14. Arch Ophthalmol 2012;130:12:1517–24.
3. Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: The Beaver Dam Eye Study. T Am Ophthal Soc 2000;98:133–41; discussion 141-3.
4. Ehlers JP, Fekrat S. Retinal vein occlusion: Beyond the acute event. Surv Ophthalmol 2011;56:4:281–99.
5. Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia: The Blue Mountains Eye Study. Arch Ophthalmol 1996;114:10:1243–7.
6. Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: The Blue Mountains Eye Study. Arch Ophthalmol 2006;124:5:726–32.
7. Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010;117:2:313-319.
8. Klein R, Moss SE, Meuer SM, Klein BEK. The 15-year cumulative incidence of retinal vein occlusion: The Beaver Dam Eye Study. Arch Ophthalmol 2008;126:4:513–8.
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol 1994;117:4:429–41.
10. [No authors listed] Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol 1997;115:4:486–91.
11. Sperduto RD, Hiller R, Chew E, et al. Risk factors for hemiretinal vein occlusion: comparison with risk factors for central and branch retinal vein occlusion: The eye disease case-control study. Ophthalmology 1998;105:5:765–71.
12. Bertelsen M, Linneberg A, Christoffersen N, Vorum H, Gade E, Larsen M. Mortality in patients with central retinal vein occlusion. Ophthalmology 2014;121:3:637–42.
13. O’Mahoney PRA, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:5:692–9.
14. Stem MS, Talwar N, Comer GM, Stein JD. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology. 2013;120:2:362–70.
15. Chen Y-Y, Sheu S-J, Hu H-Y, Chu D, Chou P. Association between retinal vein occlusion and an increased risk of acute myocardial infarction: A nationwide population-based follow-up study. Plos One 2017;12:9:e0184016.
16. Hu C-C, Ho J-D, Lin H-C. Retinal vein occlusion and the risk of acute myocardial infraction: A 3-year follow-up study. Brit J Ophthalmol 2009;93:6:717.
17. Ho J, Liou S-W, Lin H-C. Retinal vein occlusion and the risk of stroke development: A five-year follow-up study. Am J Ophthalmol 2009;147:2:283-290.
18. Janssen M, Heijer M den, Cruysberg J, Wollersheim H, Bredie S. Retinal vein occlusion: A form of venous thrombosis or a complication of atherosclerosis? Thromb Haemostasis 2005;93:06:1021–6.
19. Vessey MP, Hannaford P, Mant J, Painter R, Frith P, Chappel D. Oral contraception and eye disease: Findings in two large cohort studies. Brit J Ophthalmol 1998;82:5:538.
20. David R, Zangwill L, Badarna M, Yassur Y. Epidemiology of retinal vein occlusion and its association with glaucoma and increased intraocular pressure. Ophthalmologica 1988;197:2:69–74.
21. Fong ACO, Schatz H. Central retinal vein occlusion in young adults. Surv Ophthalmol 1993;37:6:393–417.
22. Lahey JM, Tunç M, Kearney J, et al. Laboratory evaluation of hypercoagulable states in patients with central retinal vein occlusion who are less than 56 years of age. Ophthalmology 2002;109:1:126–31.
23. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology 2011;118:1:119-133.
24. Weinberg D, Dodwell DG, Fern SA. Anatomy of arteriovenous crossings in branch retinal vein occlusion. Am J Ophthalmol 1990;109:3:298–302.
25. Zhao J, Sastry SM, Sperduto RD, et al. Arteriovenous crossing patterns in branch retinal vein occlusion. Ophthalmology 1993;100:3:423–8.
26. Group TCVOS. A randomized clinical trial of early panretinal photocoagulation for ischermic central vein occlusion: The Central Vein Occlusion Study Group N Report. Ophthalmology 1995;102:10:1434–44.
27. Glacet-Bernard A, Coscas G, Chabanel A, Zourdani A, Lelong F, Samama MM. Prognostic factors for retinal vein occlusion: A prospective study of 175 cases. Ophthalmology 1996;103:4:551–60.
28. [No authors listed] Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion: A randomized clinical trial. Arch Ophthalmol 1986;104:1:34–41.
29. Clarkson JG, Chuang E, Gass D, et al. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion: The Central Vein Occlusion Study Group M Report. Ophthalmology 1995;102:10:1425–33.
30. Fekrat S, Juan E de. Chorioretinal venous anastomosis for central retinal vein occlusion: Transvitreal venipuncture. Ophthalmic Surg Lasers Imaging Retin 1999;30:1:52–5.
31. McAllister IL, Gillies ME, Smithies LA, Rochtchina E, Harper CA, Daniell MD, et al. The Central Retinal Vein Bypass Study: A trial of laser-induced chorioretinal venous anastomosis for central retinal vein occlusion. Ophthalmology 2010;117:5:954–65.
32. Feltgen N, Junker B, Agostini H, Hansen LL. Retinal endovascular lysis in ischemic central retinal vein occlusion: One-year results of a pilot study. Ophthalmology 2007;114:4:716-723.
33. Arevalo JF, Garcia RA, Wu L, et al. Radial optic neurotomy for central retinal vein occlusion. Retina 2008;28:8:1044–52.
34. Spaide RF. Peripheral areas of nonperfusion in treated central retinal vein occlusion as imaged by wide-field fluorescein angiography. Retina 2011;31:5:829–37.
35. Campochiaro PA, Hafiz G, Mir TA, et al. Scatter photocoagulation does not reduce macular edema or treatment burden in patients with retinal vein occlusion: The RELATE trial. Ophthalmology 2015;122:7:1426–37.
36. Wykoff CC, Ou WC, Wang R, et al. Peripheral laser for recalcitrant macular edema owing to retinal vein occlusion: The WAVE trial. Ophthalmology 2017;124:6:919–21.
37. Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 5. Arch Ophthalmol 2009;127:9:1101–14.
38. Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 6. Arch Ophthalmol 2009;127:9:1115–28.
39. Haller JA, Bandello F, Belfort R, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology 2011;118:12:2453–60.
40. Hoerauf H, Feltgen N, Weiss C, et al. Clinical efficacy and safety of ranibizumab versus dexamethasone for central retinal vein occlusion (COMRADE C): A European label study. Am J Ophthalmol 2016;169:258–67.
41. Hattenbach L, Feltgen N, Bertelmann T, et al. Head‐to‐head comparison of ranibizumab PRN versus single‐dose dexamethasone for branch retinal vein occlusion (COMRADE‐B). Acta Ophthalmol 2018;96:1:e10–8.
42. Sharareh B, Gallemore R, Taban M, Onishi S, Wallsh J. Recalcitrant macular edema after intravitreal bevacizumab is responsive to an intravitreal dexamethasone implant in retinal vein occlusion. Retina 2013;33:6:1227–31.
43. Ozkok A, Saleh OA, Sigford DK, Heroman JW, Schaal S. The OMAR study. Retina 2015;35:7:1393–400.
44. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: Twelve-month outcomes of a Phase III study. Ophthalmology 2011;118:10:2041–9.
45. Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-Month outcomes of a Phase III study. Ophthalmology 2011;118:8:1594–602.
46. Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions long-term follow-up in the HORIZON trial. Ophthalmology 2012;119:4:802–9.
47. Epstein DL, Algvere PV, Wendt G von, Seregard S, Kvanta A. Benefit from bevacizumab for macular edema in central retinal vein occlusion: Twelve-month results of a prospective, randomized study. Ophthalmology 2012;119:12:2587–91.
48. Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: The RETAIN study. Ophthalmology 2014;121:1:209–19.
49. Campochiaro PA, Wykoff CC, Singer M, et al. Monthly versus as-needed ranibizumab injections in patients with retinal vein occlusion: The SHORE study. Ophthalmology 2014;121:12:2432–42.
50. Heier JS, Clark WL, Boyer DS, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: Two-year results from the COPERNICUS study. Ophthalmology 2014;121:7:1414-1420.
51. Korobelnik J-F, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: One-year results of the Phase 3 GALILEO study. Ophthalmology 2014;121:1:202–8.
52. Narayanan R, Panchal B, Das T, et al. A randomised, double-masked, controlled study of the efficacy and safety of intravitreal bevacizumab versus ranibizumab in the treatment of macular oedema due to branch retinal vein occlusion: MARVEL Report No. 1. Brit J Ophthalmol 2015;99:7:954.
53. Clark WL, Boyer DS, Heier JS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology 2016;123:2:330–6.
54. Larsen M, Waldstein SM, Priglinger S, et al. Sustained benefits from ranibizumab for central retinal vein occlusion with macular edema: 24-month results of the CRYSTAL study. Ophthalmol Retin 2018;2:2:134–42.
55. Narayanan R, Panchal B, Stewart MW, et al. Grid laser with modified pro re nata injection of bevacizumab and ranibizumab in macular edema due to branch retinal vein occlusion: MARVEL report no 2. Clin Ophthalmol Auckl N Z 2016;10:1023–9.
56. Miwa Y, Muraoka Y, Osaka R, et al. Ranibizumab for macular edema after branch retinal vein occlusion. Retina 2017;37:4:702–9.
57. Ashraf M, Souka AAR, Singh RP. Central retinal vein occlusion: Modifying current treatment protocols. Eye 2016;30:4:505–14.
58. Pfau M, Fassnacht-Riederle H, Becker MD, Graf N, Michels S. Clinical outcome after switching therapy from ranibizumab and/or bevacizumab to aflibercept in central retinal vein occlusion. Ophthalmic Res 2015;54:3:150–6.
59. Papakostas TD, Lim L, Zyl T van, et al. Intravitreal aflibercept for macular oedema secondary to central retinal vein occlusion in patients with prior treatment with bevacizumab or ranibizumab. Eye 2016;30:1:79–84.
60. Wirth MA, Becker MD, Graf N, Michels S. Aflibercept in branch retinal vein occlusion as second line therapy: clinical outcome 12 months after changing treatment from bevacizumab/ranibizumab—A pilot study. Int J Retin Vitreous 2016;2:1:20.
61. Novartis reports one year results of Phase III MERLIN study evaluating Beovu every four week dosing and provides update on Beovu clinical program. https://www.novartis.com/news/media-releases/novartis-reports-one-year-results-phase-iii-merlin-study-evaluating-beovu-every-four-week-dosing-and-provides-update-beovu-clinical-program. [Accessed November 1, 2021]
62. Clearside Biomedical announces SAPPHIRE Phase 3 study of combination therapy in retinal vein occlusion did not meet its primary endpoint. https://ir.clearsidebio.com/news-releases/news-release-details/clearside-biomedical-announces-sapphire-phase-3-study. [Accessed November 1, 2021]
63. FDA accepts application for Genentech’s faricimab for the treatment of wet age-related macular degeneration (AMD) and diabetic macular edema (DME). https://www.gene.com/media/press-releases/14923/2021-07-28/fda-accepts-application-for-genentechs-f. [Accessed November 9, 2021]
64. Kodiak Sciences treats first patients in three Ph. 3 studies of KSI-301 – Two studies in DME and one study in macular edema due to RVO. https://kodiak.com/press-releases/kodiak-sciences-treats-first-patients-in-three-ph-3-studies-of-ksi-301-two-studies-in-dme-and-one-study-in-macular-edema-due-to-rvo/. [Accessed November 1, 2021]



