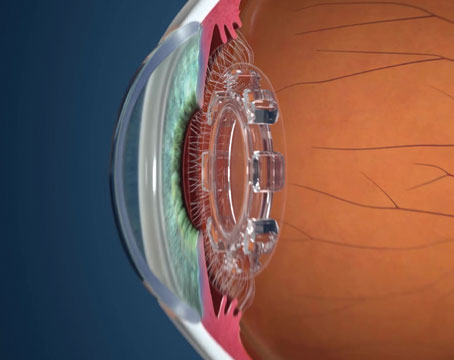
The heidelberg retina tomograph is known for its posterior-segment applications, but it will soon be able to image the anterior segment by way of a corneal module. Here's a look at this new modification to the HRT-II and what it may be able to offer the corneal specialist or anterior-segment surgeon.
The Cornea Module is a combination of software and hardware that's added to the HRT-II to increase its functionality. The hardware change involves a permanent modification to the chin rest, a pull-down forehead rest and a new, 6-inch long microscope lens that's fitted to the front of the device. It provides black and white images at 600X magnification with a resolution of 1 µm.
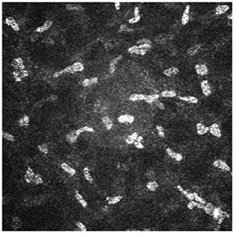 |
| Mid-cornea section at 300 µm shows keratocytes on the HRT-II Cornea Module. |
Though it hasn't yet received 510(k) clearance for marketing from the U.S. Food and Drug Administration, it eventually will be sold as a standalone unit or as a modification to a practice's existing HRT-II.
William Mathers, MD, professor of ophthalmology at the Casey Eye Institute at Oregon Health and Sciences University, uses other confocal scanning instruments to study the cornea, and is eagerly awaiting the chance to put the HRT's Cornea Module through its paces in studying corneal inflammation and other conditions.
"We've always known that confocal microscopy can optically reveal what is there," he says. "But the previous instruments haven't totally fulfilled that potential, mostly because of various technical limitations, such as camera function, tolerable brightness and the need for video capture. The HRT-II still has video capture, but because it uses laser light, the images I've seen from it suggest that it may achieve a higher resolution and can see what we haven't seen before but know is there." The Cornea Module can apparently visualize epithelial cells, keratocytes and endothelial cells, as well as identify micro-organisms such as Acanthamoeba in vivo.
"We could always see Acanthamoeba organisms, fungi, inflammatory cells and yeast particles on the surface of the eye," Dr. Mathers explains. "But it's sometimes very difficult to distinguish one from the other. We're hoping that, with better resolution, we'd be able to do that more definitively. We'll also be able to get a better view of the corneal nerves as they exist in pathologic states and when they regrow."
The new device may not be for everyone, however, though Dr. Mathers can envision clinical uses as well as research-oriented ones.
"It isn't just a research tool," he says. "Though I don't think a solo practitioner in general practice would want one, a large group with cornea, retina and glaucoma subspecialties could use it. For instance, in a corneal practice, a patient may have herpes but you might not be sure. You could culture the eye, or send away for a PCR to diagnose the virus, but that can take some time and cultures aren't always very good. Being able to use an instrument to differentiate a viral process from something else is very useful."
Though pricing is purely speculative as the company waits for marketing clearance, Heidelberg's vice president, John Hawley, says that if a user bought an HRT-II for just the cornea application, it would most likely cost just over $50,000. To modify an existing unit for the cornea application might cost $22,500.