If retinal surgery were easy, there’d be no need to constantly strive to enhance its safety and precision. Unfortunately, the delicate nature of the retinal tissue and the limits of our innate human dexterity and tactile sensitivity have led researchers to explore robotic options that might yield safer and even more effective results for our patients. Here, we take a look at the retinal procedures that could be performed best by robotic surgery systems, the systems that are currently in development and the obstacles still standing in the way of all-robot surgical procedures.
Why Robots?
Retina surgery requires high precision due to the tissue’s micro-dimensional tissue structure. The retina itself is around 250 to 300 µm thick, retinal membranes are between 20 to 40 µm, and retinal vein diameters are approximately 120 to 200 µm.1 Since human hand tremor is approximately 200 to 350 µm in amplitude,2 this surgery requires intense concentration from surgeons to avoid the risk of intraoperative complications.
What’s more, the manipulation force involved in retina surgeries is often below our tactile perception. In one study, for instance, only about 20 percent of surgeons were able to detect the forces measured during retina surgeries.3 The inability to adequately observe and control the forces results in the potential for tissue damage and other surgical complications.
From a clinical point of view, instrument operation through a pivot point is inverted and non-intuitive for human surgeons. Involuntary stress will be applied on the sclera if the instrument isn’t pivoted exactly at the incision site, which increases the difficulty for a surgeon to precisely perform retina surgeries.
Because of these areas for potential problems, some common retinal procedures have received considerable attention from researchers and robotic engineers. These surgeries include epi-retinal membrane peeling, subretinal injection and retinal vein occlusion treatment.
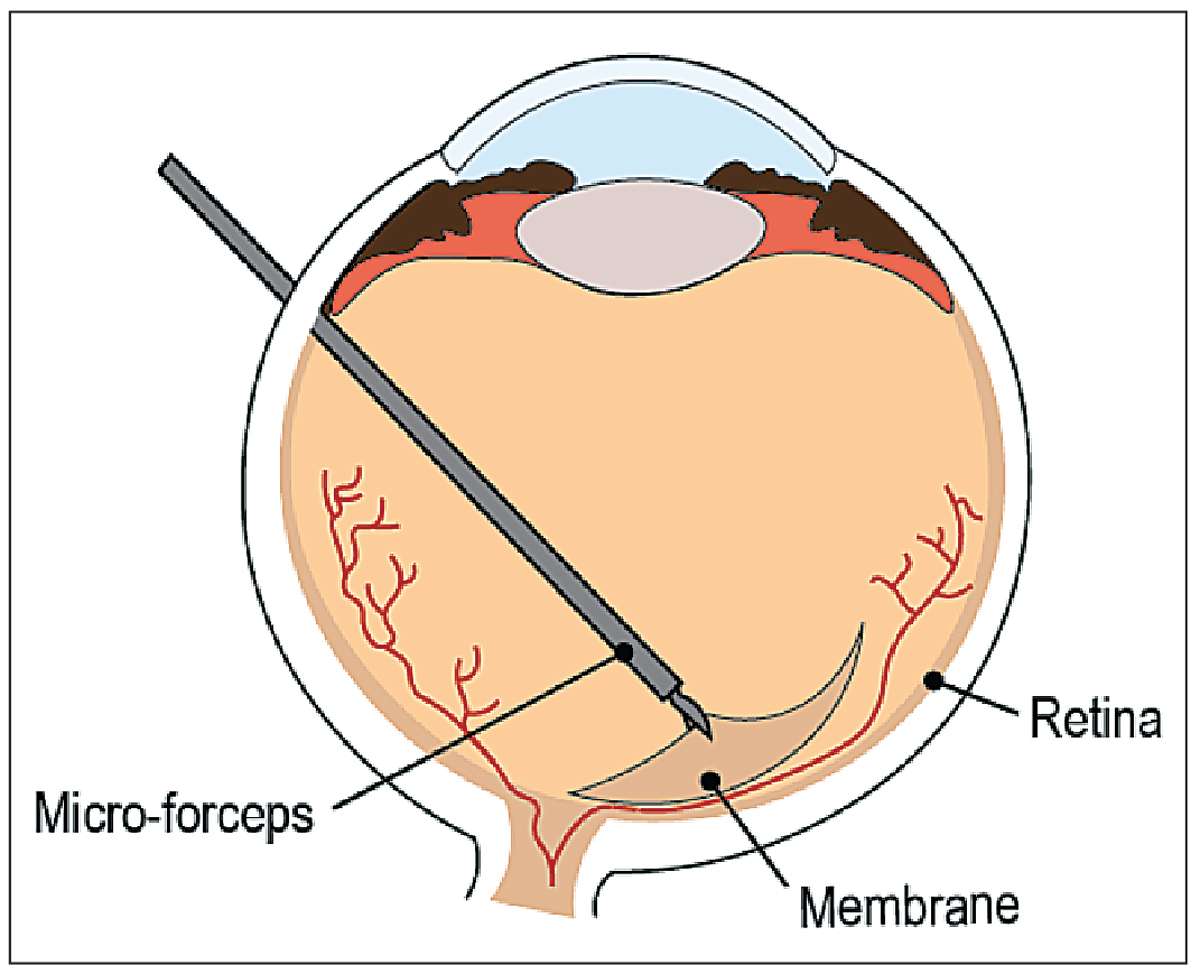 |
| Figure 1. Epiretinal membrane peeling is a target of robotic systems. |
• ERM peeling. An epiretinal membrane is a fibrocellular proliferation that can form on the inner surface of the retina, with its risk of occurrence rising significantly with age.1 Although asymptomatic when the membrane is translucent and thin, the traction on the retina that occurs as it thickens may cause macular distortion and loss of central vision function.4 The prevalence of ERM is 2 percent in individuals under age 60 and 21 percent in those over age 70.5 Typical treatment includes pars-plana vitrectomy followed by ERM peeling (See Figure 1) which requires precise manipulation of a layer that’s, on average, 61 ±28 µm thick6 to remove the retinal traction. The peeling of the additional layer (limiting membrane) will result in reducing the recurrence of the epiretinal membrane.
• Subretinal injection. This may be an alternative treatment for neovascularization, and many consider it to be the most effective delivery method for gene and stem-cell therapy because most disease processes affect the cell types in the outer retina regions.7 Current technology can penetrate the outer retina and inject the therapeutic agents in the subretinal space during a vitrectomy. However, subretinal injection risks include retinal detachment, vitreous hemorrhage, and damage of the optic nerve.8 These are often associated with patient motion, the surgeon’s hand tremor, and limited visualization of the intraocular environment.9
• RVO treatment. Retinal vein occlusion is one of the most common causes of retinal vascular abnormality in adults and a frequent cause of visual loss,10 and is strongly associated with the age of the patient. Treatment options are available but there is no permanent cure. Retinal vein cannulation (Figure 2) is a potential remedy for RVO in which an anticoagulant is injected into the retinal veins and dissolves the occlusion. However, this remains a theoretical solution due to our physiological limitations, such as hand tremor and limited depth perception.
State-of-the-Art Robotic Systems
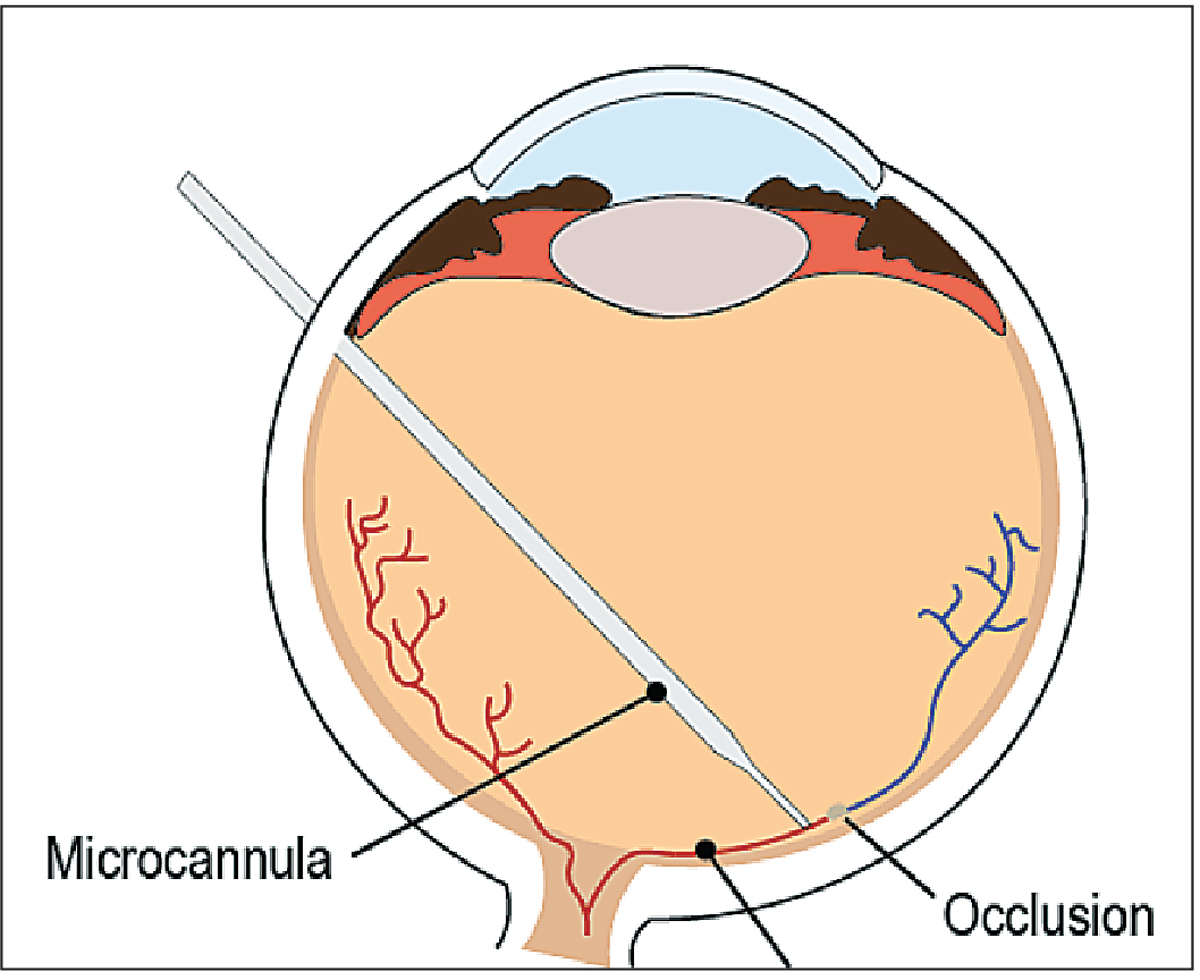 |
| Figure 2. Illustration of retinal vein cannulation, one of the procedures for which robotic systems have been used. |
Robotic systems aren’t constrained to the aforementioned human limitations because they substantially reduce human hand tremor and exhibit superior manipulation of surgical instruments. What’s more, the integration of visualization technology such as optical coherence tomography and digital microscopy can increase a robotic system’s depth perception via micrometer-level OCT axial resolution and the ability to adjust the focus on the fly. Additionally, force-sensing modalities have been integrated into surgical instruments11 that enable a better tactile sense for the user and improve surgical safety for retinal procedures. When these features are added together, the efficacy, efficiency and safety of robotic retina surgeries is enhanced, enabling such systems to autonomously perform well-defined, routine tasks. Surgeons can benefit from the use of such systems due to the robots’ incorporation of tactile feedback and visual overlays.
In an effort to overcome the potential difficulties of retinal surgery, researchers have explored the use of four types of robots: handheld; teleoperated; comanipulated; and partially or fully automated. Here’s a discussion of these various approaches.
• Handheld. These robots offer a compact, portable solution for retinal surgery. Handheld systems are readily integrated into the normal surgical workflow, as the surgeons manipulate the robot in the same manner as they would a conventional instrument. One example is the Micron system developed at Carnegie Mellon University. This three-degrees-of-freedom actuation micromanipulator increases precision via tremor compensation and anisotropic motion scaling (meaning that the micromanipulator transfers only a fraction of the surgeon’s hand motion to the tool tip).12 Because the frequency of hand tremor ranges between 8 and 12 Hz, the Micron system provides tremor compensation via a low-pass filter with a cutoff frequency of 1.5 Hz; this attenuates high frequency movements and produces a smooth motion output. Additional precision is provided using the motion scaling referred to earlier. Experimental results were obtained by having surgeons attempt to perform retinal vein cannulation, both with and without the use of the handheld Micron. The result was an increase in the success rate from 29 to 63 percent using unaided and aided surgery, respectively.13
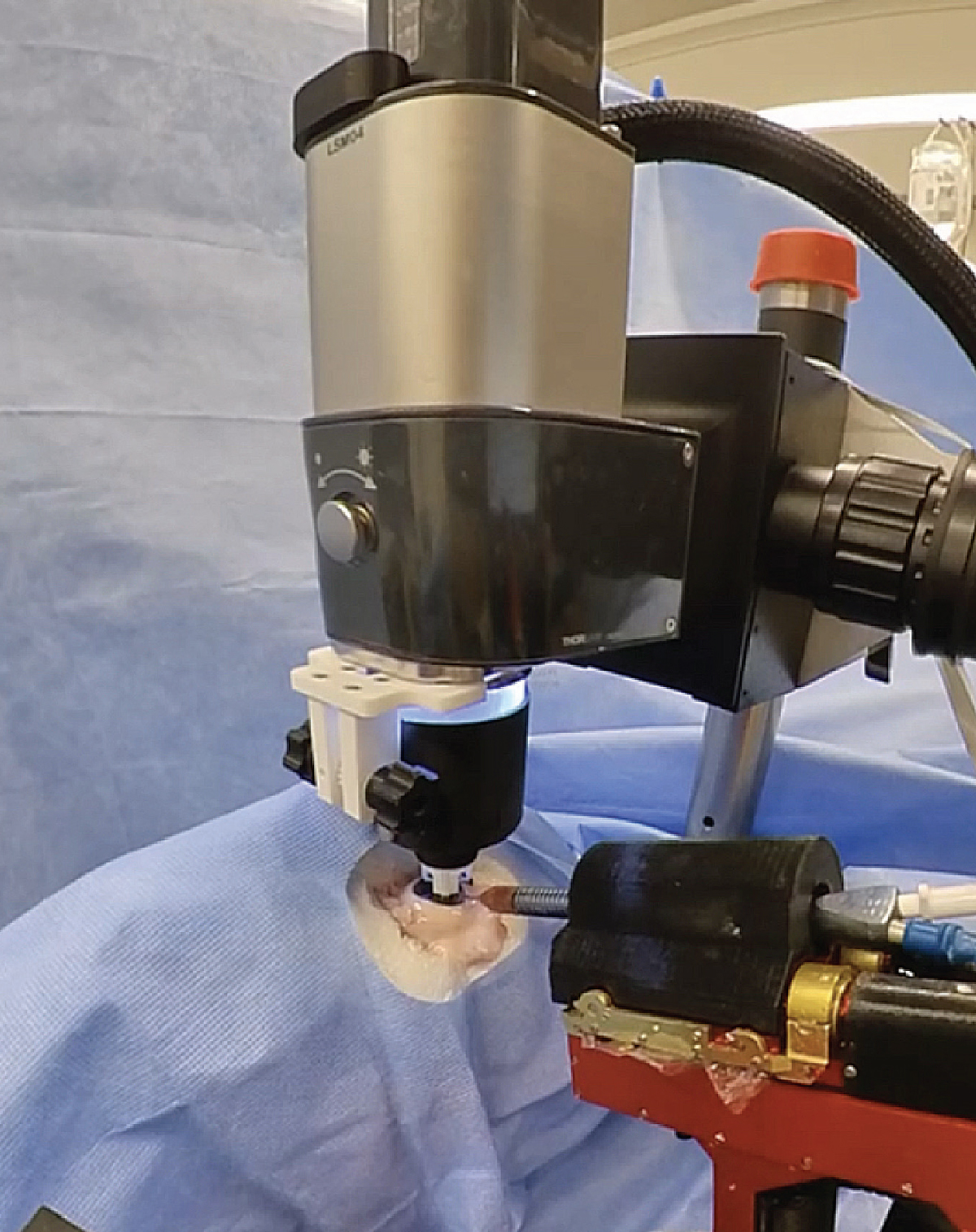 |
| Figure 3. The Intraocular Robotic Interventional Surgical System, developed at UCLA, is integrated with an optical coherence tomography system. |
The Micron handheld robot has also been used to perform preliminary tests of epiretinal membrane peeling.14 To track the position of the tool near the retina, three LEDs were mounted on the tool handle. The system locates the LEDs with position-sensitive detectors using a custom-built optical tracking system. Also mounted on the Micron is a laser to track the location and orientation of the retinal plane. The designers also implemented two virtual fixtures: motion-scaling to limit tool motion perpendicular to the estimated retinal plane and a hard stop to prevent the tool from penetrating the retina below a certain depth.
Micron also uses “velocity scaling,” which limits the motion of the tool tip to 1 mm/s, reducing the likelihood of retinal tearing during the peeling process. The system was tested on artificial phantoms consisting of a plastic film on a rubber pad. In a trial on 16 phantoms, the Micron system enabled successful adherence to the hard stop, as well as a 43.49-percent reduction in maximum engaging force and a 43.7-percent reduction in peeling force.
• Tele-operated. Teleoperated systems allow surgeons to perform operations from long distances via wired or wireless connections. The surgeon is stationed at a controller site and controls the motion of a remote robot that performs the surgical operation, monitoring the procedure using visual feedback. This remote method of surgery offers the surgeon more operating space and dexterity, and the robotic system provides enhanced precision beyond the surgeon’s capabilities.
One example of a teleoperated surgical robot is a unit developed at the University of Tokyo.15 The micromanipulator on the robot side has circumscribed degrees of freedom, moving along spherical guides, and is limited to inserting and pulling motions.
The input motion at the controller side is scaled from 40 to 1 on the robot side, providing increased accuracy of the motion. The robot side also includes what’s known as a “remote center of motion,” which means that the robot is designed to maintain a fixed pivot point. When this pivot point on the robot is aligned with the scleral incision, for instance, the surgical tool enters the eye through this port and exerts no lateral stress on the cornea. On the surgeon side, he sees a three-dimensional, high-definition view of the surgical scene presented on a 6-inch liquid crystal display monitor with a higher resolution than conventional monitors.
The system was tested by comparing the surgeons’ accuracy in performing retinal vein cannulation on custom retina models, with and without the system. The success rate for drug injection increased from 47 percent without the system to 94 percent with the robot.16
Another tele-operated system is the Intraocular Robotic Interventional Surgical System (IRISS), which was developed here at UCLA’s Advanced Robotic Eye Research laboratory as a collaboration between the Jules Stein Eye Institute and the engineering and computer vision departments.2,17 The robot consists of two independently controllable arms that slide along a circular track and can interface with two surgical tools to perform bimanual surgical tasks. On the control side, the surgeon receives visual feedback via a TrueVision 3D surgical camera18 and performs the surgery remotely by manipulating two controllers, the motion of which is modified with appropriate filtering and scaling to reduce tremor and increase precision.19 Surgeons used the system to successfully perform three retinal tasks on four ex-vivo porcine eyes each: vitrectomy; induction of posterior vitreous detachment; and microcannulation of temporal veins without retinal tears or perforations.20
• Co-manipulation. In a co-manipulation robotic system, the operator interacts directly with the robot. Surgeons have expressed particular interest in these systems because the operator retains control of the surgical tool, which some say results in a more intuitive system.21 An additional benefit is that co-manipulation robots have a smaller footprint in the operating room compared to teleoperation systems.
Belgium’s University of Leuven developed a successful co-manipulation robot called Mynutia.21 The creators say that the system provides stability to the eye by limiting the degrees of freedom of the instrument from six to four. Moreover, the motion is constrained around a remote center of motion, preventing rotation of the eye during incision. Throughout the surgical procedure, the co-manipulation system enhances the precision of the surgeon’s motion by providing motion-opposing forces with magnitudes that increase with the speed of motion. These forces attenuate the involuntary instrument motion that arises from hand tremor, enabling a steady approach towards the retina, the designers say. In January of 2017, the Mynutia system was used to perform the first safe, successful robot-assisted retinal vein cannulation in a human eye with an RVO.
• Partially or fully automated. In an automated robotic system, the surgery is performed mostly, or entirely, by the robot, which some argue offers a significant improvement in the accuracy and precision of retinal surgery compared to humans.
The IRISS system has also been used to demonstrate partial automation of retinal surgery (See Figure 3). To enable automation, a Thorlabs OCT imaging system and full-color camera were integrated into the system.2 This imaging system provides visual feedback to the robot throughout the surgical procedure to help guide its trajectory through tissue.
To validate the use of IRISS for retinal surgery, surgeons used it to perform retinal vein cannulation on custom vein phantoms.2 In practice, the user first acquires the dimensions and geometry of the silicone phantom via an OCT volume scan. They then select a desired cannulation site in the camera view, and the robot generates an approach trajectory to guide the micropipette safely through the incision, using visual cues to perform vein cannulation. In the study, the system demonstrated successful infusion in 30 trials with vein phantoms. Current research involves updating the IRISS system to perform retinal vein cannulation autonomously on ex-vivo pig eyes.
Future Applications
Vitreoretinal surgeries that aren’t feasible for surgeons may benefit from the increased stability, accuracy and enhanced sensing capabilities of a robotic system. Besides the advancement of robots and surgical tools, microscope-integrated OCT provides real-time OCT image data overlaid with a microscopic view of the surgical field. Additional sensing modalities using stereo cameras can also be incorporated into either robotic systems or existing microscope systems to enhance depth perception during the surgery.
Gene and stem-cell therapeutic treatments are currently experiencing significant progress in treating severe retinal disorders, with visual acuity improving in more than half of the eyes treated in one study.22 However, the treatment needs to be delivered between subretinal layers, which requires micrometer-level instrument operation and enhanced tool stability, especially in the presence of eye motion. While this type of sub- retinal injection can result in complications such as retinal detachment, vitreous hemorrhage and postoperative choroidal neovascularization, robotic systems have the potential to increase the accuracy and stability of the treatment’s delivery beyond human capabilities.
In the near future, robotic systems may be able to perform fully automated procedures without the input of a surgeon. Such capabilities require improved visualization, superior acquisition quality, increased speed and better interpretation of OCT or other imaging data. As the development of more robust and accurate segmentation techniques progresses, the robotic systems will have better knowledge of the vitreoretinal workspace and “no-fly zones” in the eye, enabling automation through closed-loop and real-time control.
In addition, augmented-reality imaging can be added to robotic retinal surgery systems.23 Such a system is equipped with multi-sensory feedback through a unified interface that allows the surgeon to sit comfortably while manipulating a pair of joysticks. A range of visual, haptic and auditory feedback can be integrated into the system and provide the surgeon with key information at each step of the surgery. This type of system could be beneficial in a complex retinal case such as dissecting epiretinal tissue that requires accurate and bimanual operation. By overlaying high-level membrane dissection planes atop a zoomed-in visualization of the retinal environment, the surgeon can make use of information shown on the screen that’s not currently provided in our ORs.
In the distant future, we envision robotic surgical systems capable of making surgical decisions and performing the operation’s steps without any human intervention. These systems would also be able to perform automatic tool exchanges to accomplish different phases of the procedure. Although such a system isn’t currently used in actual clinical practice, the underlying technologies that could bring this vision into reality are being developed at various research institutes throughout the world.
In conclusion, robotic surgical systems have the potential to be more accurate and safer than human surgeons when handling delicate retinal tissues. Such systems remain an active area of research, and their future use depends on the outcomes of clinical trials. Although the systems employed by these research groups have demonstrated promising results for retina surgeries, several challenges remain before they’ll be useful enough for fully automated surgeries. Engineers and surgeons are currently hard at work on ways to clear these final hurdles.
Mr. Lai is currently pursuing a PhD in adaptive and iterative learning control with the Mechanical and Aerospace Engineering Department at the University of California-Los Angeles.
Ms. Reyes is pursuing a PhD in mechanical engineering, with a focus on systems and control, at UCLA.
Dr. Tsao is a professor in the Mechanical and Aerospace Engineering Department at UCLA. They can be reached at yutingkevinlai@ucla.edu, miareyes10@ucla.edu and ttsao@ucla.edu.
Dr. Hubschman is a professor of ophthalmology in the retina division of UCLA’s Stein Eye Institute, and a professor of Mechanical and Aerospace Engineering at UCLA. He can be reached at hubschman@jsei.ucla.edu.
None of the authors have any financial interest in any devices mentioned in the article.
1. Vander Poorten E, Riviere CN, Abbott JJ, et al. Robotic retinal surgery. In: Abedin-Nasab M, ed. Handbook of Robotic and Image-Guided Surgery. Amsterdam: Elsevier, 2020:627–672.
2. Gerber M. Optical coherence tomography–guided robotic system for automated retinal microsurgery. University of California, Los Angeles, 2019 (open-access e-article).
3. Gupta PK, Jensen PS deJuan E. Surgical forces and tactile perception during retinal microsurgery. In: Taylor C, Colchester A, eds. Medical Image Computing and Computer-Assisted Intervention – MICCAI’99. MICCAI 1999. Lecture Notes in Computer Science, vol 1679. Berlin, Heidelberg: Springer, 1999:1218–1225. https://doi.org/10.1007/10704282_132.
4. Bu SC, Kuijer R, X.-R. Li, et al. Idiopathic epiretinal membrane. Retina 2014;34:12:2317–2335.
5. Mitchell P, Smith W, T. Chey, et al. Prevalence and associations of epiretinal membranes: The Blue Mountains Eye Study, Australia. Ophthalmology 1997;104:6:1033–1040.
6. Wilkins JR, Puliafito CA, Hee MR, et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology 1996;103:12:2142–2151.
7. Stout JT, Francis PJ. Surgical approaches to gene and stem cell therapy for retinal disease. Human gene therapy 2011;22:5:531–535.
8. Peng Y, Tang L, Zhou Y. Subretinal injection: A review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic research 2017;58:4:217–226.
9. Lee S, Kang JU. CNN-based CP-OCT sensor integrated with a subretinal injector for retinal boundary tracking and injection guidance. Journal of Biomedical Optics 2021;26:6:068001.
10. Rogers S, McIntosh RL, Cheung N, I. E. D. Consortium, et al. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010;117:2:313–319.
11. Ourak M, Smits J, Esteveny L, et al. Combined OCT distance and FBG force sensing cannulation needle for retinal vein cannulation: In vivo animal validation. International journal of computer assisted radiology and surgery 2019;14:2:301–309.
12. Cuevas Tabares J, Maclachlan RA, Ettensohn CA, et al. Cell micromanipulation with an active handheld micromanipulator. Ann Int Conf IEEE Eng Med Biol Soc 2010;4363-6.
13. Becker BC, Voros S, Lobes LA, et al. Retinal vessel cannulation with an image-guided handheld robot. Program of the the 2010 EEE EMBS meeting;5420–5423. https://www.ri.cmu.edu/pub_
14. Routray A. Towards retinal membrane peeling with a handheld robotic instrument (Masters thesis) 2019. https://www.ri.cmu.edu/wp-
15. Ueta T, Yamaguchi Y, Shirakawa Y, et al. Robot-assisted vitreoretinal surgery. J Ophthalmol;116:8:1538–1543.
16. Tanaka S, Harada K, Ida Y. Quantitative assessment of manual and robotic microcannulation for eye surgery using new eye model. Int J Med Robot, 2015;11:210–217.
17. Summary of research. https://www.uclahealth.org/
18. Product information, TrueVision 3D. https://www.leica-
19. Wilson JT, Gerber MJ, Prince SW, et al. Intraocular robotic interventional surgical system (IRISS): Mechanical design, evaluation, and master–slave manipulation. International Journal of Medical Robotics and Computer Assisted Surgery, 2018;14:1.
20. Rahimy E, Wilson J, Tsao TC, et al. Robot-assisted intraocular surgery: Development of the IRISS and feasibility studies in an animal model. Eye (Basingstoke) 2013;27:8: 972–978.
21. Gijbels A, Smits J, Schoevaerdts L, et al. In-human robot-assisted retinal vein cannulation, a world first. Annals of Biomedical Engineering, 2018;46:10:1676–1685.
22. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label Phase I/II studies. The Lancet, 2015;385:9967:509–516.
23. Gerber MJ, Pettenkofer M, J.-P. Hubschman. Advanced robotic surgical systems in ophthalmology. Eye 2020;34:9:1554–1562.



