When it comes to Acanthamoeba keratitis, the best treatment is prevention. These insidious amoebae are found worldwide,1 in virtually every environment, including soil, air, swimming pools, bottled water, domestic tap water and, most importantly for our purposes, contact lens solutions and paraphernalia.
Diagnosing this condition can be tricky—and treatment even more so—as the little buggers are highly resistant to conventional antimicrobial therapies, as well as freezing, desiccation and the typical chlorine levels used for drinking water disinfection.2 Here, we’ll discuss ocular Acanthamoeba infection, its epidemiology, clinical presentations and the latest methods of diagnosing and treating this insidious threat.
Themes and Variations
 |
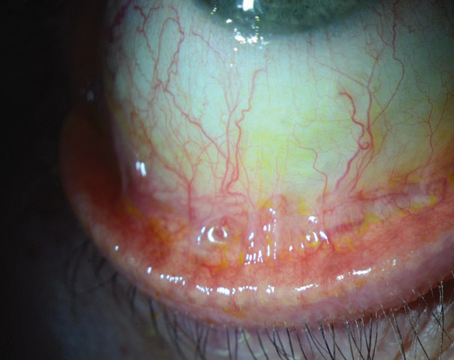
Though Acanthamoeba keratitis is relatively rare, accounting for perhaps 5 to 10 percent of all microbial keratitis, at referral centers like Tufts New England Eye Center we en-counter at least one new case per month (Figure 1). The first case of Acanthamoeba in the eye was reported 1973, in a South Texas rancher with a history of right eye trauma.2 Subsequently, with increasing use of soft contact lenses and FDA approval of extended-wear contact lenses, Acanthamoeba keratitis cases began to mount; probably 85 to 90 percent of Acanthamoeba keratitis is contact lens-associated, with incidence estimates of about one corneal infection in 10,000 contact lens wearers per year,3-6 and increasing.
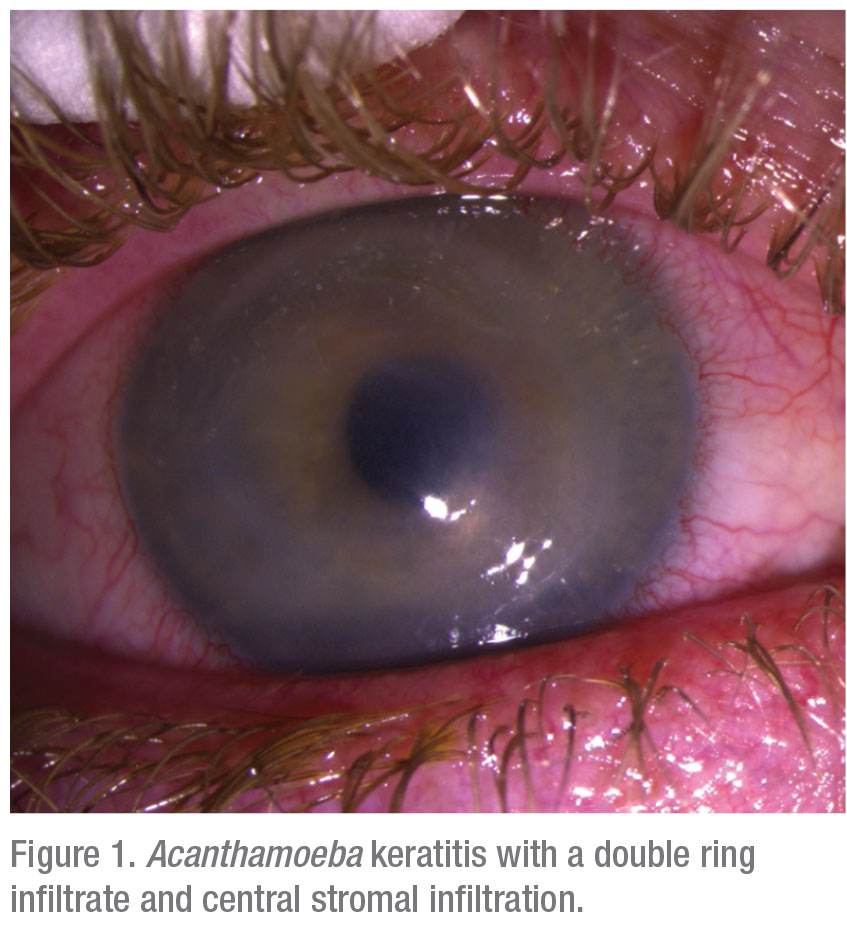
Acanthamoeba is a genus of free-living bacterivore amoebae. They’re able to inhabit every conceivable form of water source and supply and also have the especially confounding ability to morph between two evolutive forms: an active trophozoite form and a dormant cyst (Figure 2A). When faced with adverse environmental conditions, such as cold temperatures or antibiotic therapy, the Acanthamoeba trophozoite assumes its cystic form, a 15- to 30-µm sphere, which is largely impervious to stress and can maintain viability for years. The active and reproductive trophozoites, usually 25 to 50 µm in size7 are often overlooked in microscopic smears and sections because of their resemblance to white blood cells.
An Ounce of Prevention
Given the nearly ubiquitous presence of Acanthamoeba in water—whether fresh water, sea water, tap water, bottled water, swimming pools or hot tubs, we always advise patients to remove contact lenses for showering and swimming, especially in fresh water.
There was an Acanthamoeba keratitis epidemic in the 1980s after the FDA approved the making of saline solution from salt tablets for contact lens hygiene. Instead of using sterile distilled water, lens wearers would cut corners and just throw in some tap water, with predictably disastrous results. Then as now, poor contact lens hygiene—using home-brewed saline, storing lenses in tap water, rinsing lens cases in tap water—remains a major contributor to corneal infection. And even if proper care methods are observed, the extended wear of contact lenses overnight and use of orthokeratology lenses are additional risk factors.3 Even among non-contact lens wearers, ocular exposure to contaminated water supplies while swimming, hot tubbing or even aquarium cleaning can result in Acanthamoeba infection.
 |
The Great Masquerader
While most microbial keratitis, even among contact lens wearers, remains bacterial, the seemingly straightforward corneal infection that doesn’t respond to usual regimens of antibiotics must be suspected for the less common causative organisms, be they fungal, viral and/or protozoal. Hence, although the community standard of care remains treating with a fluoroquinolone antibiotic, usually in the absence of corneal smears and cultures, the recalcitrant cases persisting after perhaps five to seven days of treatment are suspect for the rarer infection sources and even polymicrobial infection. Co-infection with bacteria or herpes simplex virus can occur in 10 to 23 percent of keratitis cases.8
In such antibiotic-unresponsive scenarios, and given the con-founding effect of antibiotic therapy on establishing a diagnosis, the cases which are referred to specialized centers must not only be corneal cultured and occasionally biopsied but, importantly, all of the patient’s medications and topical eyedrops—especially contact lenses, cases and solutions—should be cultured, given the high probability of revealing the hidden or masquerading organism (Figure 2B).7
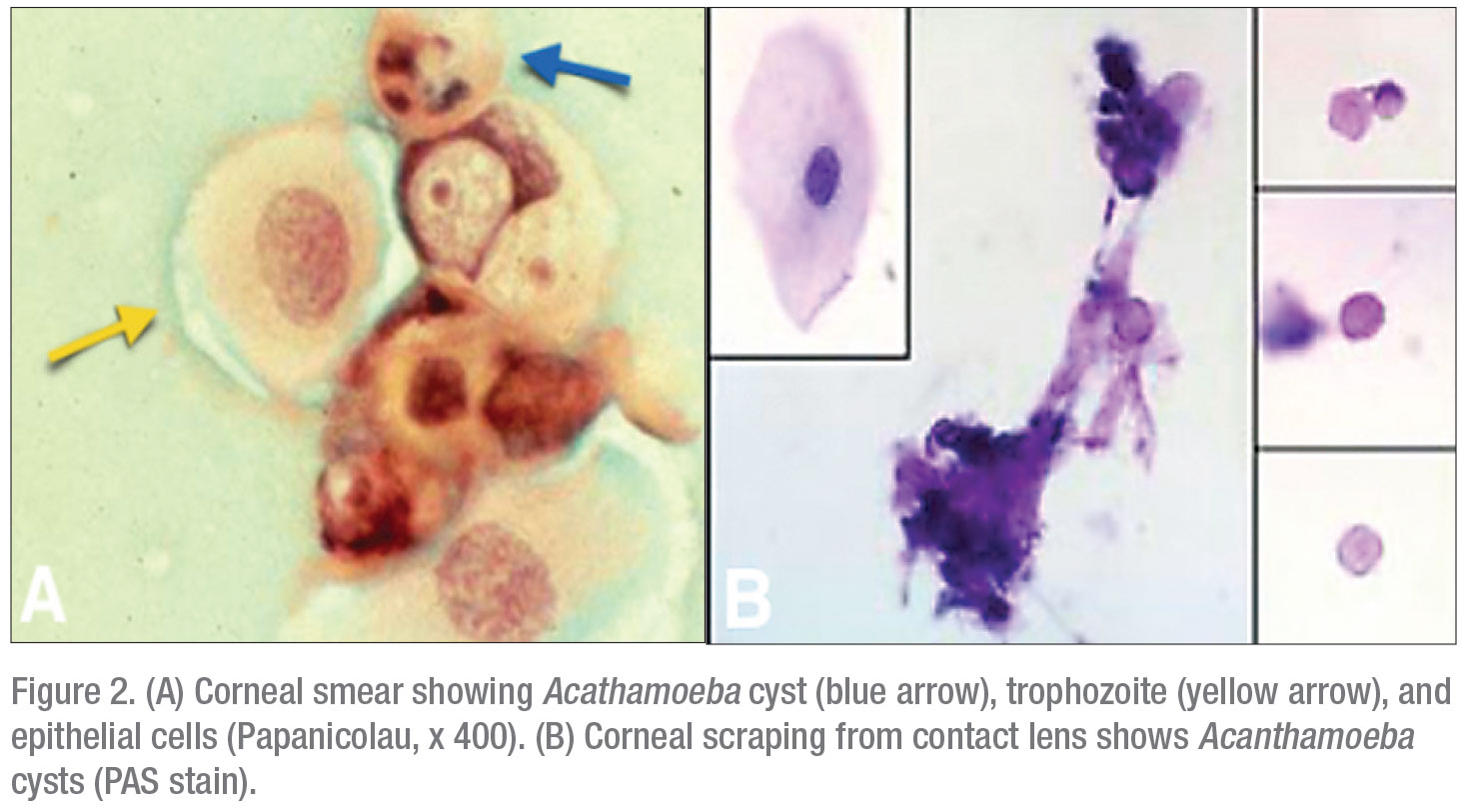
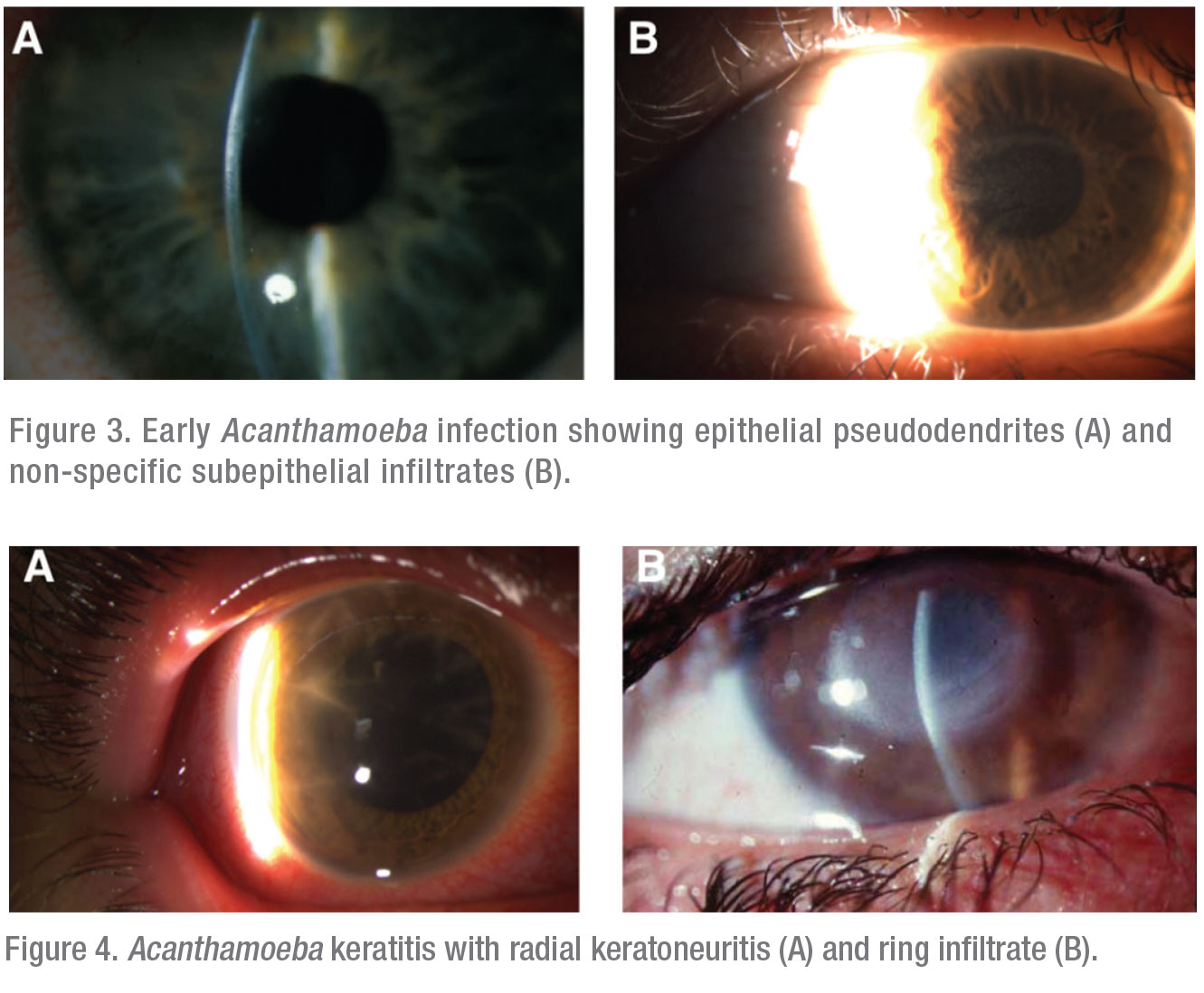
The typical clinical presentations of Acanthamoeba keratitis exhibit one or more of three hallmark features. In increasing severity, the first is an epithelial pseudodendrite, which can resemble lesions caused by HSV (Figure 3A) or subepithelial infiltrates (Figure 3B). The second is radial keratoneuritis, developing along inflamed corneal nerves in a web-like pattern (Figure 4A). The third and most advanced is the ring infiltrate, resembling a Life Saver candy—a white ring of intrastromal inflammatory cells with a clear center (Figure 4B).
As Acanthamoebae invade the corneal stroma, they elicit a massive inflammatory response of white blood cells which, in their effort to eradicate the infection, inflict their own collateral damage, resulting in a loss of corneal clarity and enzymatic digestion of corneal tissue (stromalysis). Moreover, since the inflammatory process involves corneal sensory nerves, it seemingly elicits such excruciating ocular pain that patients often confine themselves to darkened rooms due to extreme photophobia.7
Given the resistance of the infection and the chronicity of the inflammatory response, therapy, once it’s initiated, is continued until complete microbial eradication is achieved, a process that can often take months. Even if the infection doesn’t extend beyond the cornea to involve the sclera, the inflammatory consequences of limbal stem cell deficiency, cataract, iris atrophy and secondary glaucoma (consequent to PAS) further compromise the ocular surface and anterior segment.
Complex Diagnostics
For all referred infectious keratitis cases, especially those non-responsive to customary antibiotic therapies, it becomes mandatory to go back to square one and perform the full battery of corneal scrapings and cultures, including the customized approach for those suspect for Acanthamoeba.9
 |
In particular, corneal scrapings must be not only stained with Gram, Giemsa and PAS, but also stained with calcofluor white, acridine orange and immunospecific antibodies, as well as routine PAS.3,10 Moreover, cultures require special growth conditions—specifically an E. coli-enriched medium, which must be observed for at least one week.
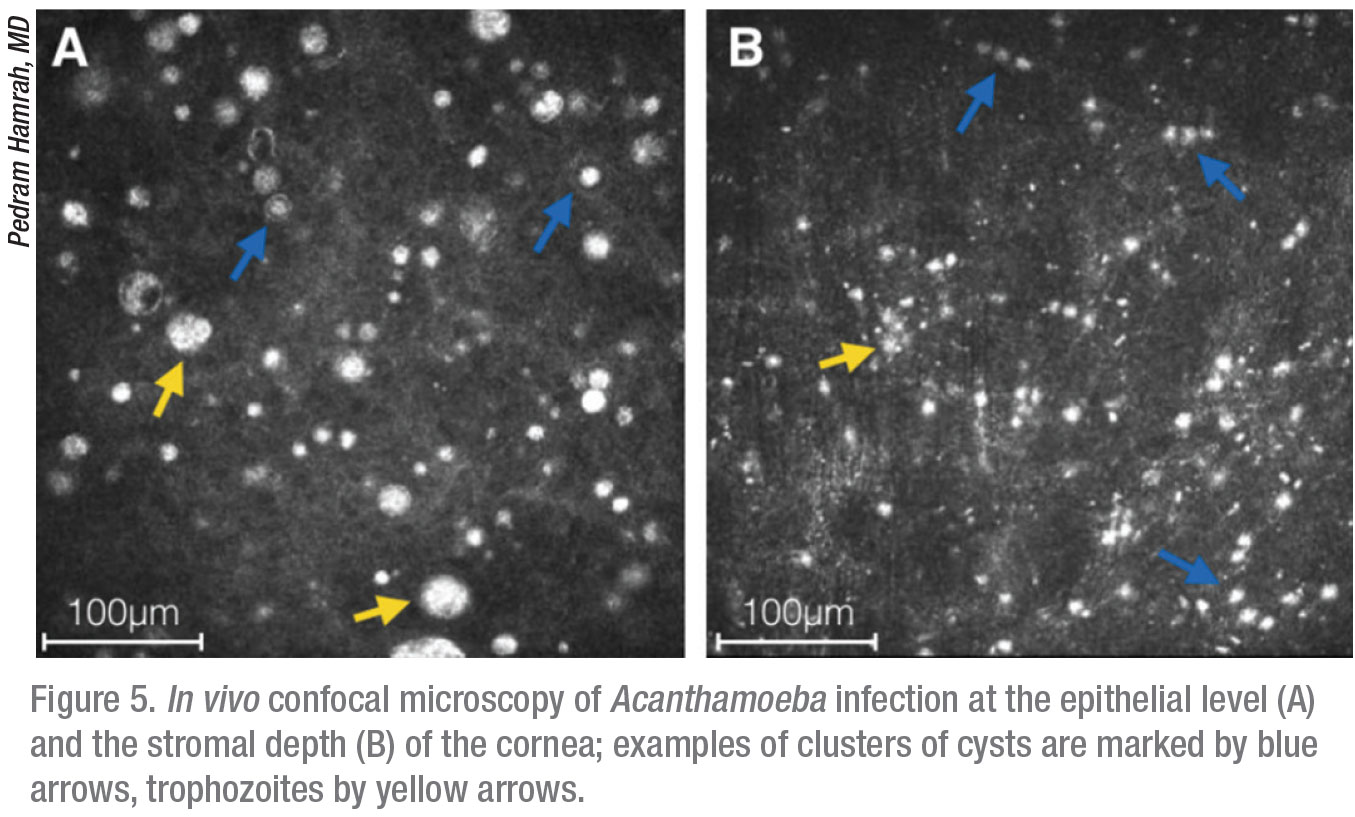
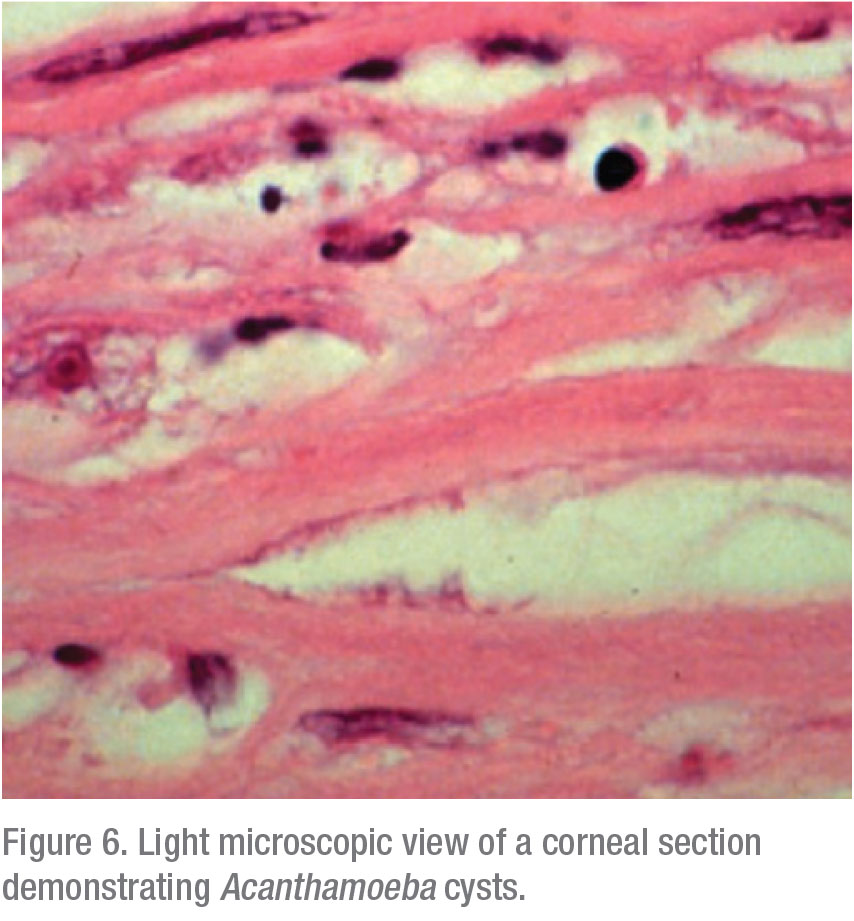
Apart from these traditional microbiological modalities for diagnosis, the confocal microscope offers in vivo imaging of the affected cornea at magnification of up to 800x, thereby directly resolving the presence of infectious organisms. This laser microscope is non-invasive and affords rapid diagnosis of Acanthamoeba and/or other infections at earlier stages, when treatment is more effective. We’re able to see into the depths of the cornea at a very high magnification and obtain microstructural imaging at the cellular level; cysts are characteristically reflective and round, whereas trophozoites exhibit hyperreflective ovoid or irregular profiles (Figure 6).11,12
Although the microscope’s small field of view and high magnification can be misleading and result in a false positive or negative diagnosis,13 in experienced hands, it’s very helpful for differentiating between the trophozoite and cystic forms and, more importantly, for localizing the depth of their invasion. We can also use it as a monitoring device to track response to therapy, to guide the tapering of medications after symptom resolution and to detect co-infection by other microbes.
Tough Treatment
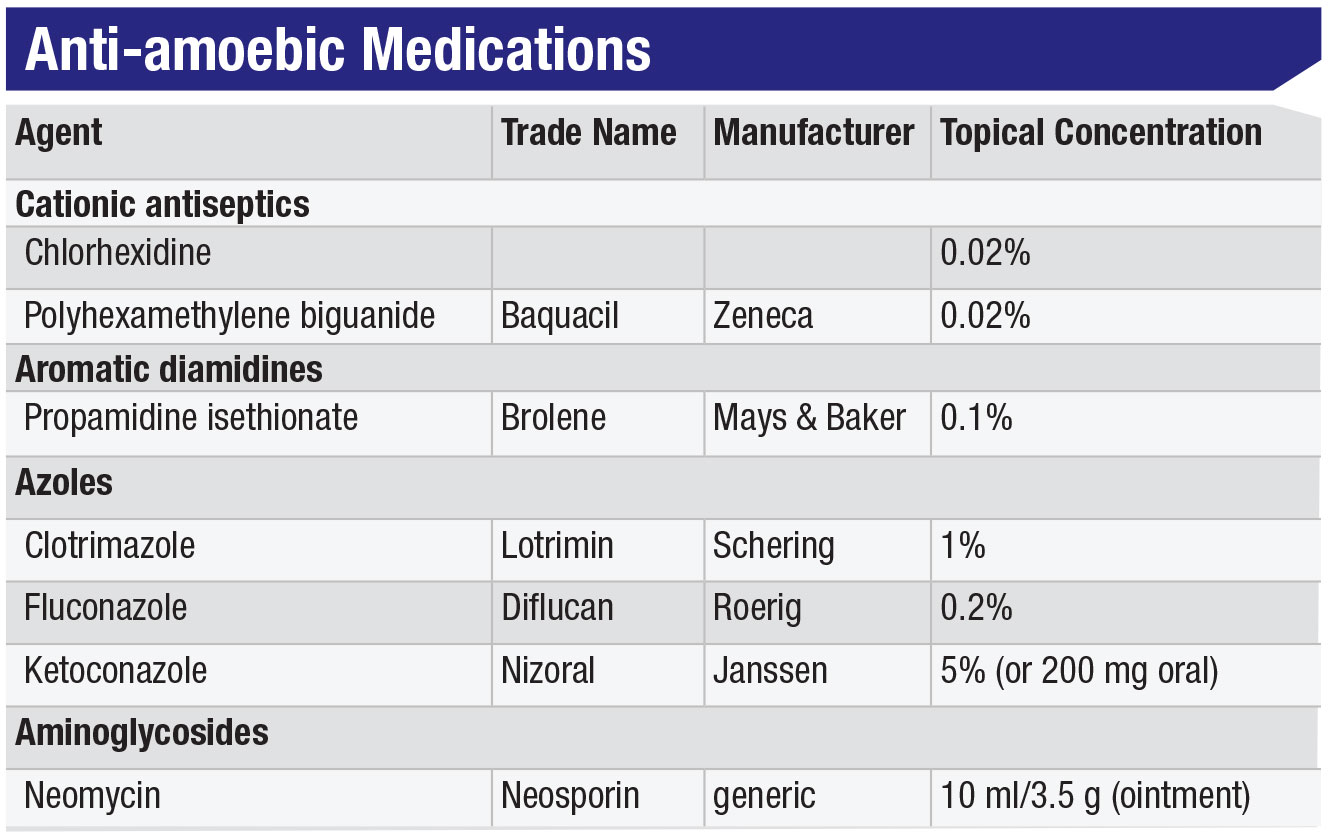
If Acanthamoeba keratitis is difficult to diagnose, it’s even tougher to treat. Potentially requiring months to eradicate, Acanthamoeba infection is very challenging for both the patient and the clinician. For starters, Acanthamoeba doesn’t respond to antivirals or most antibiotics. The various classes of amoebicidal drugs (see Table, below), are difficult to obtain (most being non-FDA approved), challenging to formulate and toxic to use, and therefore difficult for patients to tolerate during the long course of treatment.
Of the agents that are useful for killing the trophozoites, there are three groups: 1) topical biguanides, including chlorhexidine 0.02% and polyhexamethyline biguanide (PHMB), which are, in fact, swimming pool cleaners; 2) topical diamidines, of which propamidine or Brolene 0.1% are most commonly used;3,14 and 3) various antifungal imidazoles, such as fluconazole, ketoconazole and voriconazole, in both topical and/or oral routes of administration (see Table).3 The diamidines interfere with the amoebae’s DNA synthesis but, while they’re OTC in Europe, they’re not available in the United States. We typically use some cocktail combination of biguanide, diamidine and/or imidazole, depending on clinician preference, drug availability and patient tolerance.
In contrast, Acanthamoeba cysts are essentially “bulletproof,” remaining impervious to amoebicial drugs. The somewhat controversial treatment strategy of using topical steroids is beneficial for preventing the drug-susceptible trophozoites from conversion to drug-resistant cysts. However, the steroid balancing act is delicate, since the trophozoites also remain reproductive and steroids will suppress the immune and inflammatory responses, possibly facilitating additional stromal ulceration. Here again, the confocal microscope is extremely valuable for determining the state of the amoebic organisms’ concentration and viability, and for assessing their activity.
 |
Play to Win
Acanthamoeba keratitis usually presents unilaterally with both the trophozoite and cystic forms present, and as soon as it’s diagnosed it must be treated very aggressively, according to the previously specified regimen. Even a mild, superficial infection can progress rapidly, and the deeper into the cornea the amoebae invade, the more inaccessible they become to poorly penetrating topical drugs. Since trophozoites replicate quickly, it’s important to apply drugs hourly around-the-clock for at least the first 48 hours. Nighttime drug administration can then be reduced, while daytime drops remain hourly. Based on the therapeutic response, and as the infection subsides, the medication frequency can be slowly tapered to perhaps four times a day, which often must be maintained for several months.3
 |
The goal of treatment is to kill all trophozoites and cysts, as evidenced by clinical and confocal microscopic improvement. As previously discussed, judicious use of topical steroids may be beneficial both to lure trophozoites out of encystment and to control the chronic inflammatory process. Meanwhile, be sure to constantly manage ocular surface drug toxicity and allergy, neurotrophic persistent epithelial defects, and intraocular pressure as the inflammation subsides.
Another therapeutic strategy of potential, but equivocal, benefit is corneal cross-linking (CXL), utilizing much the same techniques that have been developed for keratoconus stabilization. Presumably the ultraviolet light exposure of CXL is effective for killing organisms located relatively superficially, but with less efficacy for deeper-penetrated amoebae.7,9 As results remain somewhat inconsistent, however, further investigation is required to refine the CXL technique for this use.
 |
Surgical interventions may be required during active therapy, ranging from corneal debridement of non-viable tissue to aid drug penetration, to amniotic membrane disc or graft application to reduce inflammation and promote epithelial recovery, to therapeutic keratoplasty for anatomical restoration of extremely thinned or perforated corneas. The latter also reduces the intrastromal microorganism load and prevents extension infection to the limbus and sclera, whereupon it becomes nearly impossible to eradicate. Emergent glaucoma surgery and extraction of intumescent cataract may also be required.
Following eradication of active infection, anatomical and visual rehabilitation might require limbal autograft transplantation to restore the damaged limbal stem cell population; medical treatment of neurotrophic keratopathy with nerve growth factor (Oxervate, Dompé); definitive cataract, glaucoma and/or anterior segment reconstructive surgery; and, once all associated issues have been resolved, penetrating keratoplasty to replace the scarred cornea.
After all is said and done, the visual prognosis for most medically treated patients is on the order of 20/100. For eyes requiring penetrating keratoplasty, the risk factors of chronic inflammation, stromal neovasularization, limbal deficiency, glaucoma and prior keratoplasty all affect the prognosis, but overall keratoplasty survival is approximately 50 percent. Among successful grafts, there’s a 50-percent chance of attaining 20/40 vision.15 As with other high-risk keratoplasties, management of immune rejection, ocular surface issues and intraocular pressure continues indefinitely.7,9
Parting Pearls
Following are some tips for particular aspects of managing Acanthamoeba:
• An ounce of prevention… As primary vision providers, educate contact lens wearers about the perils of poor lens hygiene, extended and over-extended contact lens wear and ocular water exposure while wearing contacts.
• Red-flag alert. Just as any acute contact-lens-related keratitis should be considered to be Pseudo-monas until proven otherwise, any CL-related keratitis unresponsive to one week of conventional and/or fortified topical antibiotic therapy should be considered to be Acanthamoeba, fungal and/or viral and must undergo more rigorous diagnostic culture and imaging studies to verify an exact etiologic diagnosis. Above all, don’t delay by playing polypharmacy roulette: Referral for an appropriate evaluation and management should be on a “run, don’t walk” basis, since any delay vastly prolongs and worsens the prognosis.
• Confirm etiology. As the Acanthamoeba therapy train rolls on forever, specific confirmation of diagnosis by culture, imaging and/or biopsy is mandatory. Remember: Acanthamoeba, fungus and even viral infections can produce clinically similar keratitis, so there is seldom justification for continuing empirical “shotgun therapy.” Once diagnostically confirmed, therapy has to be “all-in”: aggressive, with multiple agents, intensive topical application and recognition of potential medication toxicity, given the months of therapy required for Acanthamoeba eradication.
• “It ain’t over ’til it’s over.” Finally, given the long-term course of medical therapy plus the associated aspects of ocular surface and other anterior segment sequelae, the commitment of both clinicians and patients becomes a “BFF” (best friend forever) relationship, recognizing that following eradication of the infection, the subsequent medical and surgical management requires equally committed and vigilant commitment.
In conclusion, Acanthamoeba keratitis, particularly among highly at-risk contact lens wearers, remains the most difficult of corneal infections to diagnose and treat. Maintaining a high level of suspicion for Acanthamoeba as the potential etiology for recalcitrant keratitis cases, referral for diagnostic studies and aggressive pharmacologic management are mandatory to save such eyes from devastating visual loss. Although medical therapy is intense, toxic and seemingly interminable, medical cures are nonetheless attainable. If the “collateral damage” of ocular surface, cornea, cataract and glaucoma don’t prove insuperable, then anatomical and visual recovery are feasible. Truly, desperate times call for desperate measures. REVIEW
Dr. Kenyon is a clinical professor of ophthalmology at Tufts University School of Medicine/New England Eye Center and is a faculty member at Harvard Medical School and the Schepens Eye Research Institute. Dr. Binotti is a clinical research fellow at Tufts/New England Eye Center, currently investigating anterior segment imaging modalities.
1. Marciano-Cabral F, Cabral G. Acanthamoeba species as agents of disease in humans. Clin Microbiol Rev 2003;16:273-307.
2. Alizadeh H, Silvany RE, Meyer DR, et al. In vitro amoebicidal activity of propamidine and pentamidine isethionate against Acanthamoeba species and toxicity to corneal tissues. Cornea 1997;16:94-100.
3. Keenan JD, McLeod SD. Parasitic keratitis. In: Yanoff M and Duker JS, eds. Ophthalmology, 5th ed. Edinburgh, UK: Elsevier 2019:230-23.
4. Acanthamoeba keratitis multiple states, 2005–2007. MMWR Morb Mortal Wkly Rep 2007;56:21:532–534.
5. Thebpatiphat N, Hammersmith KM, Rocha FN, et al. Acanthamoeba keratitis: A parasite on the rise. Cornea 2007;26:6:701–706.
6. Dart JK, Radford CF, Minassian D, et al. Risk factors for microbial keratitis with contemporary contact lenses: A case-control study. Ophthalmol 2008;115:10:1647–1654.
7. Maycock NJR, Jayaswal R. Update on Acanthamoeba keratitis: Diagnosis, treatment and outcomes. Cornea 2016;35:713-720.
8. Dart JKG, Saw VPJ, Kilvington S. Acanthamoeba keratitis: Diagnosis and treatment update 2009. Am J Ophthalmol 2009;148:487-499.
9. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmol 2017; 124:1678-1689.
10. Rivière D, Szczebara FM, Berjeaud JM, et al. Development of a real-time PCR assay for quantification of Acanthamoeba trophozoites and cysts. J Microbiological Methods 2006;64:1:78–83.
11. Huang P, Tepelus T, Vickers LA, et al. Quantitative analysis of depth, distribution, and density of cysts in Acanthamoeba keratitis using confocal microscopy. Cornea 2017;36:927–932.
12. Goh JWY, Harrison R, Hau S, et al. Comparison of in vivo confocal microscopy, PCR and culture of corneal scrapes in the diagnosis of Acanthamoeba keratitis. Cornea 2018;37:480–485.
13. Parmar DN, Awwad ST, Petroll WM, et al. Tandem scanning confocal corneal microscopy in the diagnosis of Acanthamoeba keratitis. Ophthalmol 2006;113:538-547.
14. Alizadeh H, Silvany RE, Meyer DR, et al. In vitro amoebicidal activity of propamidine and pentamidine isethionate against Acanthamoeba species and toxicity to corneal tissues. Cornea 1997;16:1:94-100.
15. Ficker LA, Kirkness C, Wright P. Prognosis for keratoplasty in Acanthamoeba keratitis. Ophthalmology 1993;100:105–110.