Battling the Bugs
Steve A. Arshinoff MD, FRCSC, associate professor at the University of Toronto’s department of ophthalmology and vision sciences, employs intracameral injections to prevent endophthalmitis. “I was the first in the world to use intracameral moxifloxacin in 2004, and I still do,” he says.
Dr. Arshinoff’s infection-control protocol doesn’t involve any preoperative drops before the patient walks into the OR. “I think it is a really bad idea to give patients topical antibiotics for a few days preop. If you do that, microbiologically, the drug kills off all the sensitive bacteria, but it leaves the nutrients on the conjunctiva for resistant bacteria to grow,” he says. “You encourage resistant strains because you’re selecting for them by killing off the bacteria that are sensitive to the drug. When patients come to the operating room, they’re not there for long preoperatively, so then I give them topical Vigamox (Alcon, Novartis) three times over 15 minutes: It kills off all the sensitive bacteria that are there, but there isn’t enough time to grow resistant strains to colonize the ocular surface.”
Steven M. Silverstein, MD, FACS, in practice at Silverstein Eye Centers, Kansas City, Missouri, who takes a drops-only approach, notes that some causative factors are uncontrollable, regardless of prophylaxis used. “There are some factors beyond our control,” he says. “Even if patients are not asked to take any eye drops, patients can still rub their eyes, go to the bathroom without washing their hands, touch doorknobs, shake hands—all kinds of things we can’t control. People can contaminate their eyes in a way that antibiotics alone may not necessarily prevent, regardless of the route of administration.”
Dr. Silverstein also uses fluoroquinolone drops, but he has patients use them for two days prior, and then administers them three times right before surgery in the preop area. “They also get a topical NSAID for two days before surgery,” he says.
At the close of surgery, Dr. Arshinoff injects Vigamox into the anterior chamber. “I give my patients 600 micrograms diluted into 0.4 cc. We dilute basically the whole bottle of Vigamox—3 cc—and add 7 cc of BSS. The concentration is such that you inject 0.4 cc—a little less than the volume of the pseudophakic anterior chamber (0.5 cc). You get a totally safe dilution and a perfectly reasonable concentration to use,” he says, cautioning that other branded and generic moxifloxacin preparations have not been demonstrated to be safe for intraocular use. “Avelox (Merck) has a pH of 4.1 to 4.6, which is t
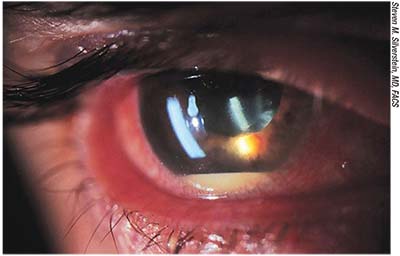 |
| Endophthalmitis with hypoyon. Most infections occur during the early postoperative period, but endophthalmitis can develop many months after cataract surgery. |
Zachary Zavodni, MD, in practice at The Eye Institute of Utah and an adjunct clinical associate professor at the John A. Moran Eye Center of the University of Utah in Salt Lake City, also gives his cataract patients intracameral Vigamox, but more often uses compounded moxifloxacin. “Intraoperatively, I have been using intracameral antibiotics for several years, as the preponderance of data supporting such a practice is compelling,” he says. “Like many surgeons, I used to use intracameral vancomycin because its coverage against gram positive bugs is so good. Approximately two years ago, however, I stopped because of the possible association between vancomycin and HORV. I now use 0.1 mL of intracameral moxifloxacin 0.05%. I inject approximately 0.05 to 0.075 mL directly into the anterior chamber at the conclusion of my case, and I use the remainder to hydrate the paracentesis wound. Depending on the setting I’m in (i.e., hospital vs. private-practice ASC) and the supply available, I will use either an unopened bottle of Vigamox or compounded moxifloxacin from an in-house compounding pharmacy or a national commercial supplier such as Imprimis or JCB Labs. Most of the time, I end up using medication compounded for intracameral use.”
Dr. Silverstein will give periocular antibiotics at the close of surgery in a select few cases. “In high-risk patients, those with vitreous loss, a prior history of endophthalmitis, or significant lid-margin disease, I will give a sub-Tenon’s injection of antibiotics, a depot injection for slow release, in conjunction with the pre- and postoperative fluoroquinolone antibiotic drops that they receive, ” he says.
Dr. Silverstein thinks the extant evidence for intracameral injections is exciting, but insufficient to change his practice pattern. “The European study was compelling enough to make us engage in serious conversation, and to look toward a new U.S.-based study with more consistent parameters,” he notes, adding that he has seen robust proposed study designs that interest him. He remains skeptical of transzonular injections. “When there is posterior capsular rupture, or even in an intact capsule, where medicine is deliberately squirted into the posterior segment through the zonular network, the potential for macular and retinal toxicity is real, especially for aminoglycosides,” he says. “Perhaps it’s not as great with the fluoroquinolones, but we haven’t really had enough experience to comment definitively about that. Without concrete data, it’s not something that I advocate at this time.”
Dr. Arshinoff says that his choice of moxifloxacin is well supported by research. “We wrote a paper fairly recently that explains why moxifloxacin is better.4 In addition, Peter Libre published a paper that demonstrated that the only drug that really kills off all the bacteria is moxifloxacin.5 I’m also currently writing a paper comparing the abatement rates of the different drugs and what happens to their concentrations, and it turns out moxifloxacin is by far the best,” he says.
Dr. Arshinoff distinguishes documented cases of uveitis-like conditions with transilluminating iris depigmentation associated with oral moxifloxacin,6 and “one or two” iritis cases arising from intravitreal delivery, from his preferred intracameral administration technique through the side-port incision at a safe concentration. “As time goes by in medicine, whenever we devise ways to give a drug directly to the area where we want it to work, it’s by far the safest way you can do it,” he says. “If you have a high systemic dose, it traverses the iris into the vitreous. It actually washes the tissues en route, where it may cause iritis. But when we put it into the anterior chamber, it’s not washing through to the iris. It’s just in the anterior chamber and the capsular bag. In a lower dose, it’s very safe,” he says.
A suspected anaphylactic response to topical moxifloxacin before cataract surgery has been documented,7 but Dr. Arshinoff says he hasn’t encountered any adverse reactions to it in intracameral use in an estimated 10,000 patients. “I’ve had patients tell me they’ve had a bad response to systemic moxifloxacin, so when they come into the operating room, I give them a drop of Vigamox while they’re waiting,” Dr. Arshinoff says. “If anything happens to them, then I won’t give them the intracameral.”
Dr. Zavodni makes exceptions to intracameral moxifloxacin use for patients with suspected fluoroquinolone sensitivities. “The only scenario in which I may vary my protocol is when a patient has a history of allergic anaphylaxis to either Betadine (Mundipharma) or fluoroquinolones. In these cases I may use topical chlorhexidine to prep the eye before surgery. As an alternative to postoperative topical fluoroquinolones, I will typically use polymyxin B sulfate/trimethoprim,” he says.
While Dr. Zavodni stopped using intracameral vancomycin because of its relatively recent association with HORV, Dr. Silverstein stopped years ago after he and his colleagues at the time determined that it didn’t appear to be effective at preventing endophthalmitis in their patients. “I was in a practice around 2000 or so, when we did a study with all of the cataract surgeons in the group to look at our practice’s endophthalmitis rates. We were using intracameral vancomycin, which was a popular product to use prophylactically at the time,” he recalls. “Looking at 9,000 cases, we saw no difference in the incidence of endophthalmitis with or without the vancomycin. We stopped using it because there was absolutely no evidence to support its use.”
Citing concerns about HORV, Dr. Arshinoff concurs that vancomycin does not belong at the forefront of endophthalmitis prophylaxis. “The risk of HORV is extremely low, but it’s an immunological condition, and often you don’t know if a patient has received vancomycin for something else before: If they were primed immunologically, then they might get a reaction to it. It’s also a drug we want to keep as a last resort,” he explains.
Postop Protocols
Ocular and periocular steroid injections—alone or together with compounded antibiotic—are part of the dropless cataract surgery trend. None of the three doctors here currently use them, however. “I think they’re a bad idea, because I think the risk lies in giving the needle. If the patient moves during that injection, it can get into their eye. I don’t think there’s enough benefit to justify doing it,” says Dr. Arshinoff, who gives Pred Forte (Allergan) drops postoperatively. “Because they only get the steroid for five to 10 days, and a pressure spike usually takes around five days to develop, I ask the patient to come back in five days. “If their pressure is high, then I will stop the Pred Forte, and I will tell them to take more ketorolac, six times a day until their 10 mL bottle is finished, and then they’re fine.”
In addition to Pred Forte and ketorolac, Dr. Arshinoff prescribes topical Vigamox postoperatively. “Those particular medications are extremely cheap in Canada: It probably costs patients $20 to get enough drops for both eyes, maybe $40. But in the U.S., it might cost a few hundred,” he says. He adds that he also gives drops because he thinks they help curb his patients’ postoperative behavior. “I do 90 percent bilateral cataract surgery. Bilateral cataract surgery patients may think that their surgery is trivial because they sit up and can see clearly right away out of both eyes. I don’t want my patients to think that their surgery is trivial: When I give them drops, they think they’d better be careful for the first few days. They don’t rub their eyes; they’re more attentive to how they’re healing and more careful overall. After the first three days, I back them off the drops from six to three or four times a day until the bottles are finished,” he says.
Dr. Zavodni doesn’t inject intracameral or intravitreal steroids at close of surgery, either. Because of their short half-life he says, “These medications require either transzonular placement or pars plana injection at the end of the case. Potential drawbacks include: possible IOP rise; rebound inflammation once the steroid is gone; and some of the injected steroids are opaque, causing increased floaters postoperatively.” Dr. Zavodni does prescribe topical drops postoperatively because they remain the standard of care. “I am still using topical fluoroquinolones for the first week after surgery. I am aware that there aren’t any randomized controlled trials to support such a practice, and that Herrinton’s retrospective study out of Kaiser Permanente in 20162 demonstrated that topical antibiotic used in conjunction with intracameral was not better than intracameral alone,” he acknowledges. “Nonetheless, the use of topical antibiotics is still considered standard of care across the United States [98 percent of ASCRS surgeons reported postoperative use of topical antibiotics in a 2014 survey], and I haven’t felt comfortable deviating until prospective data exists proving that it’s not providing any benefit. I also use both topical steroids and NSAIDs following surgery, although the efficacy of NSAIDs is highly debated.”
Dr. Silverstein’s all-drops regimen includes postoperative topical NSAIDs and steroids as well as an antibiotic. “My patients receive preoperative fluoroquinolone drops for two days before surgery, and then three times prior to surgery in the preop area. They continue postoperatively until the bottle runs out, which will get them two to three weeks of additional antibiotics. If it’s a high-risk case, I’ll supplement that with periocular gentamycin or gentamycin and Kefzol (Lilly) sub-Tenon’s,” he explains. “They get a topical NSAID for two days before surgery, on the day of, and then after surgery. If they’re not patched, they continue the NSAID till the bottle runs out, which could be two to four weeks. It’s the same with the steroids: They’ll get the steroid after surgery if they’re not patched until the bottle runs out, which will cover them for two to four weeks.”
To dropless-surgery advocates
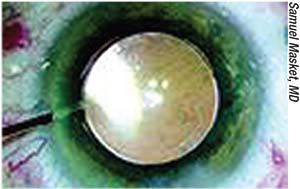 |
| Intracameral injection of an antibiotic preparation at close of surgery has been more widely adopted outside of the United States. |
“Really, the only criticism of drop therapy—until we have a definitive answer about efficacy—is compliance,” he says. “That’s really the only legitimate argument dropless proponents have that that I agree with.” To simplify drop compliance, Dr. Silverstein says that a postop nurse sits with patients and family members to review all post-surgical care and restrictions, including the drop regimen. “They’re handed a sheet that spells out the drops they should take and at what frequency,” he explains. This encounter comes after prior interventions to help patients and caregivers get a handle on topical eye drops. “From the preoperative counselors, they’ve already gotten a handout with this information on it, and a life-sized color picture of the bottles of medicine they’re going to be using. And then it’s written down for them again on postop day one, when we review the instructions and decide whether or not we want to increase or decrease the frequency of anything. If someone has a significant amount of inflammation, we may temporarily increase the frequency of the steroid, for example. If there was a capsular rupture, we may increase the frequency of the steroid and the NSAID to help prevent CME. It really is no issue whatsoever,” he states.
One alternative to the treatment burden of topical steroids recently received FDA approval. DEXYCU (Icon Bioscience Inc.; Newark, California) is an extended-release dexymethasone suspension intended for injection behind the iris and in front of the IOL at close of surgery. DEXYCU’s intracameral dose is 5 µL (equivalent dexamethasone dose: 517 µg) “The medication dissolves over three weeks and removes the need for a topical corticosteroid for most patients,” says Eric D. Donnenfeld, MD, clinical professor of ophthalmology, New York University and trustee, Dartmouth Medical School, and lead investigator for the DEXYCU clinical studies. Dr. Donnenfeld says the manufacturer hopes to make DEXYCU available sometime in 2018.
Until the Verdict Comes in
Dr. Zavodni, who uses both intracameral Vigamox and compounded preparations, summarizes the dilemma faced by surgeons who want to proceed with injections to prevent infection. “The use of intracameral antibiotics in the United States is on the rise (41 percent of ASCRS surgeons in 2017 clinical survey results),” he observes. “Yet, two large barriers to ubiquitous adoption remain: First, there is no FDA-approved antibiotic, and as a result there is no uniform/commercially produced, single-use sterile product available here. Secondly, there are safety concerns regarding medication toxicity and compounding errors.
“Currently, to use intracameral antibiotics, one must either tackle the legal and logistical challenges of preparing a medication packaged and approved for topical use in the eye (i.e., Vigamox), or rely on a compounding pharmacy to prepare such medications,” he continues. “Despite 503B status-designation standards for certain compounding pharmacies, there will always be some level of risk of dilution error and contamination, given such a production model. Concentration errors with intracameral cefuroxime have been associated with toxic anterior segment syndrome and retinal toxicity. Compared to cefuroxime, vancomycin and moxifloxacin are technically more easily compounded. However, there is concern about an association between intracameral vancomycin and HORV, which has curtailed its use in the U.S. Thus far, intracameral moxifloxacin seems to be relatively safe at standard concentrations ranging from 0.25% to 0.5%, so it has become the most popular intracameral choice of U.S. surgeons,” he says.
The “less-drops” approach—giving patients compounded fixed combinations of topical antibiotic/anti-inflammatory postoperatively, instead of separate antiobiotic, steroid, and NSAID drops—has been adopted by some surgeons attempting to decrease costs to patients and increase their compliance. One small study found a fixed-dose antibiotic/steroid drop to be just as effective at post-phaco inflammation control as an intracameral antibiotic/steroid injection, and no cases of endophthalmitis occurred in any eyes studied.8 Another compared eyes that received fixed-combination antibiotic/steroid drops with eyes receiving the components of those drops separately after cataract surgery. The fixed-dose combination was just as effective as the individual drops in preventing infection and controlling inflammation after surgery.9 One study, funded by a grant from Imprimis Pharmaceuticals, compared postop eyes that got a transzonular injection of the company’s compounded Tri-Moxi-Vanc with eyes receiving topical Pred-Moxi-Ketor drops (one week postop) followed by Pred-Ketor (two to four weeks postop). Both treatment modes appeared to have equal therapeutic benefits, but patients reported greater satisfaction with the injection.10
Dr. Silverstein says that his concerns about the sourcing and handling of ingredients deters him from using compounded
 |
| Respondents (n=83) to the e-survey published in this month’s Review of Ophthalmology showed a strong preference for topical antibiotic and anti-inflammatory drops to prevent infection and CME after cataract surgery. |
To help ensure the efficacy of what does go into his patients’ eyes, Dr. Silverstein uses only branded eye drops. “One thing that people rarely discuss is that generic steroids and NSAIDS are not as uniformly effective as their branded counterparts,” he says. “I’ll occasionally see someone come in with postop uveitis, and I’ll insist that they get off generics and switch to reliable branded topical drops.”
Dr. Arshinoff sticks to a branded agent for intraocular injection. “One problem that we have now is that all Vigamox is becoming genericized, with multiple generic versions being sold in both Canada and the U.S. So if I was going to use them—and I don’t—I would check with the company that it was the same formulation before I’d use it in someone’s eye,” he cautions. “Sandoz is owned by Alcon (Novartis) and Alcon has assured me that the Sandoz generic moxifloxacin is identical to Vigamox.”
One generally accepted component of endophthalmitis prophylaxis is povidone-iodine.11 “Everyone uses Betadine. We all clean the lashes and drape them carefully and use Betadine 10% to wash the face and 5% eye drops for at least five to 10 minutes before surgery,” says Dr. Arshinoff.
“The only thing we know to decrease periocular and perioperative bacterial load—and most likely decrease the risk of endophthalmitis and postoperative infection—is the Betadine prep around the eye and diluted drops in the eye for a few minutes before surgery. It’s the only thing we know works,” adds Dr. Silverstein.
“Nothing has proven more efficacious in preventing endophthalmitis than the use of topical povidone-iodine,” states Dr. Zavodni. “My patients receive a drop of Betadine directly into the conjunctival sac during preop and in the operating room. This is, of course, in addition to the prepping of periocular skin and lashes with Betadine before placing the surgical drapes.”
As for an FDA-approved, single-use injectable intracameral antibiotic preparation indicated for endophthalmitis prophylaxis, the prospects are not so cut and dried. “I don’t really see any company investing the money needed to have the FDA approve it. It’s just not reasonable from an economic standpoint,” says Dr. Arshinoff. “We have to look at reality, and if I were running the company, I would not be eager to go to all the expense when my drug will rapidly be genericized and the chance of recouping the investment is miniscule.”
He cites one potential off-label alternative, though. “Moxifloxacin solutions for intracameral injection that come in pre-loaded syringes are already available from a company in India (Entod Pharma). Unfortunately, they are not sold in North America. I think the company that makes it is opening a branch in the U.K., which may help to get these things available in North America. Hopefully, they will make it in the dilute formulation (to inject 0.4 mL of 150 µg per 0.1 mL) prepackaged for single use,” he says.
Even if pre-filled moxi syringes eventually become readily available, they’ll remain off-label for endophthalmitis prophylaxis, as there is no economic incentive for manufacturers to repurpose an existing generic drug, since they would be barred from having exclusive rights. “Without a foreseeable economic return, there has not been any industry incentive to push for FDA approval, which is a great detriment to our patients,” says Dr. Zavodni. “The ASCRS Research Council has thankfully begun development of a prospective research trial, which will hopefully lead to FDA approval and, ultimately, a product that all surgeons can feel confident using.”
Dr. Silverstein acknowledges that the evidence for intraocular injections to prevent endophthalmitis is growing, but he awaits tightly controlled, prospective multicenter studies to elevate the certainty of that evidence.“There are a couple of very bold and aggressive American studies being proposed now to try to answer the question definitively: Does an intracameral medication such as moxifloxacin, cefuroxime, etc., decrease the incidence of endophthalmitis? Of course, the rate-limiting factor to these kinds of studies has always been a large enough n to declare statistical significance. Thankfully, because the incidence of endophthalmitis is so low, you’d have to study many thousands of patients in order to make such a claim,” he says.
The daunting expenditures of time and money, as well as the vast statistical sample that a probative study would demand, mean that for the foreseeable future, cataract surgeons will have to continue following the succinct advice of a recent Cochrane Review when pondering antibiotics for endophthalmitis prevention: “Practitioners should rely on current evidence to make informed decisions regarding prophylaxis choices.”12 REVIEW
Dr. Silverstein has never been compensated and has no other financial interest in any of the products discussed in this article, although he has participated in research on antibiotics, topical NSAIDs and steroids throughout his career.
Dr. Arshinoff is a consultant for Alcon, but not with regard to Vigamox. He reports no other financial interests in medications mentioned in this article.
Dr. Zavodni reports no relevant financial interests.
1. ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg 2007;22:978-88.
2. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology 2016;123:287-94
3. Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin prophylaxis: Analysis of 600,000 surgeries. Ophthalmol 2017;124:6:768-75.
4. Arshinoff SA, Modabber M. Dose and administration of intracameral moxifloxacin for prophylaxis of postoperative endophthalmitis. J Cataract Refract Surg 2016;42:1730-41.
5. Libre PE, Matthews S. Endophthalmitis prophylaxis by intracameral antibiotics: In vitro model comparing vancomycin, cefuroxime, and moxifloxacin. J Cataract Refract Surg 2017; 43:833-38.
6. Knape RM, Sayyad FE, Davis JL. Moxifloxacin and bilateral acute iris transillumination. J Ophthahlmic Inflamm Infect 2013;14;3:1:10.
7. Ullman MA, Midgley KJ, Kim J, Ullman S. Anaphylactic reaction secondary to topical preoperative moxifloxacin. J Cataract Refract Surg 2016;42:1830-37.
8. Simaroj P, Sinsawad P. Effects of intracameral triamcinolone and gentamicin injections following cataract surgery. J Med Assoc Thailand 2011;94:7:819-25.
9. Cunha PA, Shinzato FA, Tecchio GT, et al. Efficacy and tolerability of a gatifloxacin/prednisolone acetate fixed combination for topical prophylaxis and control of inflammation in phacoemulsification: A 20-day double-blind comparison to its individual components. Clinics 2013;68:6:834-9.
10. Fisher BL, Potvin R. Transzonular vitreous injection vs a single drop compounded topical pharmaceutical regimen after cataract surgery. Clin Ophth 2016;10:1297-1303.
11. Speaker M G, Menikoff J A. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology 1991;98:1769–1775.
12. Gower EW, Lindsley K, Tulenko SE, Nanji AA, Leyngold I, McDonnell PJ. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No.: CD006364. DOI: 10.1002/14651858.CD006364.pub3.