Radiation retinopathy is a chronic and progressive retinal vasculopathy resulting from radiation to the orbit, globe, head or neck.1 We use the term radiation maculopathy to describe RR limited to the macula, where vascular damage leads to the most significant changes in visual acuity. RR/RM is the most common cause of severe, irreversible vision loss in eyes treated with radiotherapy.2
Here, we’ll highlight the diagnostic clues to watch for, the risk factors to take into account and the various treatment methods to consider when faced with a possible case of radiation retinopathy.
Background
While this review will primarily focus on RR caused by treatment for intraocular tumors, such as uveal melanoma, it’s crucial to recognize that radiation therapy for non-ocular neoplasms of the head, neck, and brain can also lead to this condition. In the case of intraocular tumors, we often use plaque brachytherapy, where a tiny radioactive plaque (about the size of a grain of rice) is inserted directly adjacent to the tumor. For extraocular tumors or, in some specific centers or circumstances for intraocular tumors, proton beam irradiation, delivered externally, may be the preferred treatment method.
Radiotherapy has replaced enucleation for small, medium and some large uveal melanomas since the Collaborative Ocular Melanoma Study of 1985 demonstrated there was no difference in mortality between the two treatments for medium-sized choroidal melanoma.3 As a result, radiation has become the go-to treatment, allowing us to preserve the eye, maintain useful vision and improve our patients’ quality of life.
Clinical Presentation and Diagnostics
Radiation to the orbit immediately disrupts the capillary endothelium and causes accumulation of fibrinous material within vascular walls, eventually leading to vessel occlusion and ischemia of the retina.4 In RR, retinal ischemia presents with a variable spectrum of retinal nonperfusion, retinal capillary leakage, neovascularization and hemorrhage.5 The pathology of RR is similar to diabetic retinopathy. In both diseases, vascular endothelial growth factor is released in response to ischemia and stimulates angiogenesis—or neovascularization of the retina—which poses well-known, significant problems because the newly formed vessels are disorganized, weak and susceptible to leakage. Over time, these leaky vessels lead to edema and hemorrhage, which distort the retinal architecture and lead to the visual changes seen by these patients.
Vascular damage is hypothesized to begin immediately after the retina is exposed to radiation.2,6 On average, clinical features of RR present about 32 months after treatment.7 During a dilated fundus examination, you might spot macular edema, retinal hemorrhage, microaneurysms, cotton-wool spots, hard exudates and disc swelling. While these findings are similar to diabetic retinopathy, there are a couple of key differences to keep in mind. RR is more likely to be unilateral and tends to have fewer microaneurysms. By contrast, diabetic retinopathy often lacks atrophic retinal pigment epithelium.8 Depending on the specific pathology at play, you might classify a patient’s RR as exudative (due to endothelial tight junction damage and fluid accumulation), hemorrhagic (caused by capillary weakness or abnormal neovascularization) or atrophic (characterized by thinning of the retinal pigment epithelium and retinal disorganization).5
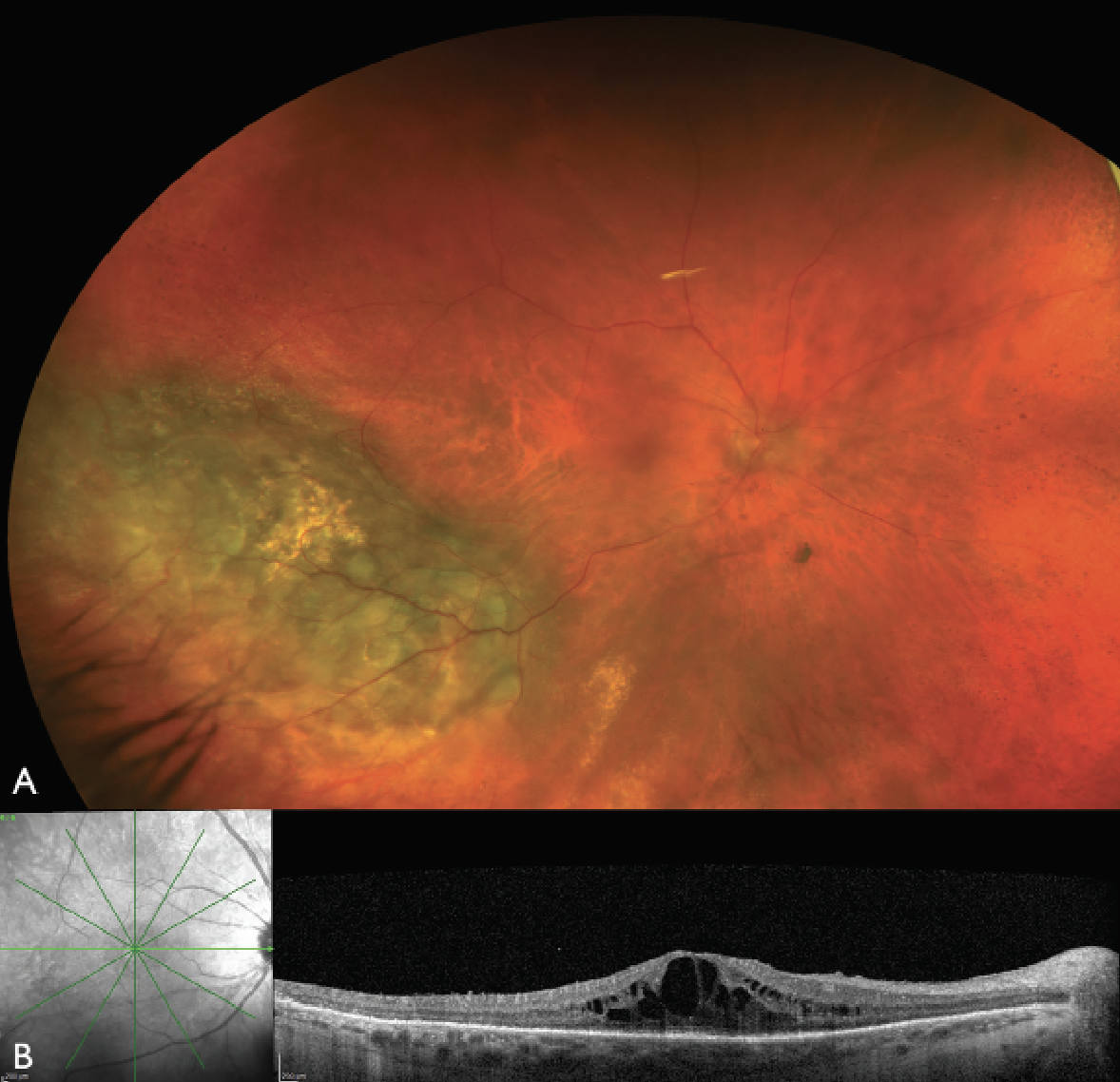 |
| Figure 1. Color fundus photograph and optical coherence tomography of the right eye one year after plaque radiotherapy for uveal melanoma. (A) The color fundus photograph reveals regressed uveal melanoma inferotemporally with no overt retinal hemorrhages or exudates typical of radiation retinopathy. (B) The OCT, however, reveals cystoid macular edema, indicating the presence of radiation maculopathy. |
Fluorescein angiography is a sensitive test for the vascular impact of RR. Given the occlusive nature of RR, capillary non-perfusion is an early and consistent sign.9 In the early 90s, a team at Queen’s Hospital Belfast developed a staging system using FA to classify RR based on microvascular changes.10 Later, the team at Wills Eye Hospital, led by Carol Shields, MD, created a classification system based on the severity of macular edema on optical coherence tomography.11 OCT has become an incredibly valuable tool in detecting early macular edema even before we can see any signs of retinopathy during a clinical exam (Figure 1). In fact, studies have shown that macular edema can be present in up to 33 percent of eyes with no clinically apparent retinopathy.8
OCT-angiography is highly sensitive to vascular changes in RR, with high definition and delineation of the retinal layers allowing for quantification of capillary disruption.5 A study from the Wills team in 2016 compared 65 patients with choroidal melanoma treated with plaque brachytherapy to non-irradiated patients. Using OCTA, they discovered enlargement of the foveal avascular zone and decreased capillary density, even in eyes without clinically evident RR.12 In another study led by Alexandre Matet, MD, of the Jules-Gonin Eye Hospital in Switzerland, 93 patients underwent OCTA imaging, and the results were striking. They found that the same features—larger foveal avascular zone and decreased capillary density—were associated with worse visual acuity.13 Since then, multiple other studies have echoed this link between OCTA and pre-clinical detection of vascular changes in RR.14,15 It’s become clear that OCTA is a sensitive tool for picking up on the subtle vascular changes that occur before we can see any clinical signs of RR.
This highlights the importance of multimodal imaging in early detection. By combining the powers of FA, OCT and OCTA, we can spot the earliest signs of RR and potentially intervene before irreversible damage occurs. As appropriate treatments are identified; this could be the key to preserving vision in irradiated eyes and giving our patients the best possible outcomes.
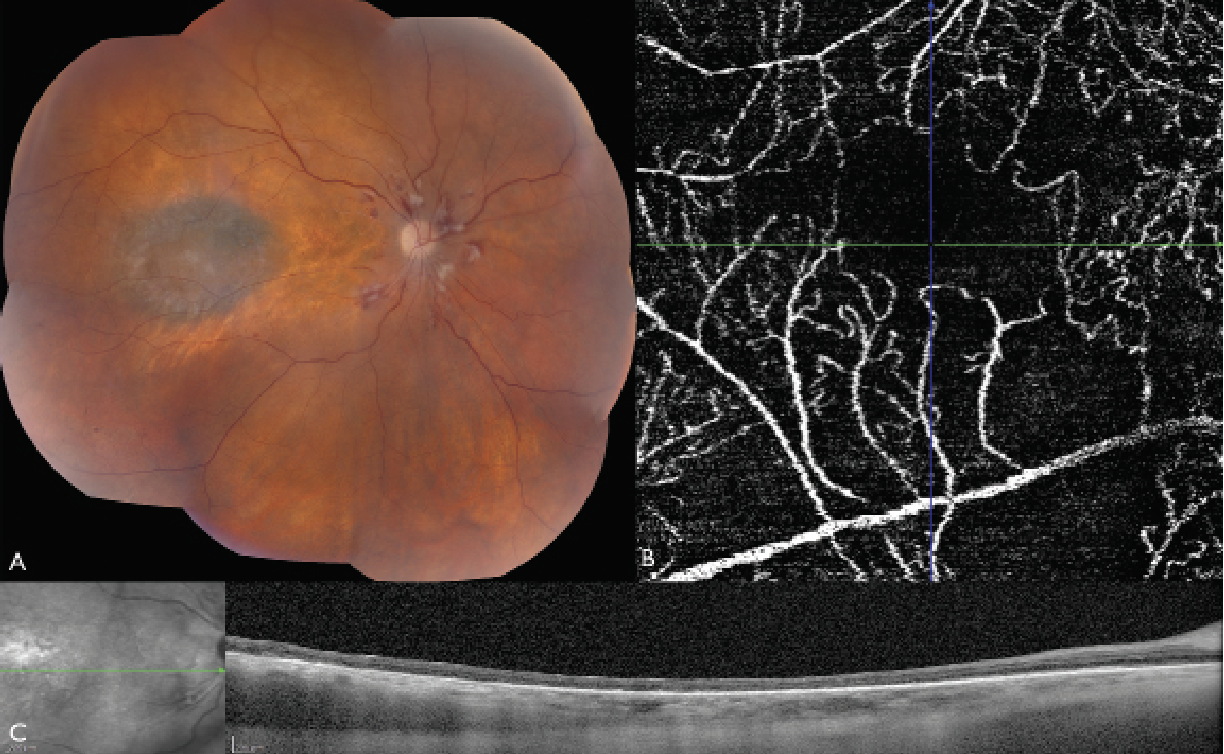 |
| Figure 2. Montage color fundus photograph, optical coherence tomography angiography, and optical coherence tomography of the right eye three years after plaque radiotherapy for uveal melanoma. (A) The color fundus photograph shows signs of atrophic radiation retinopathy and maculopathy, including retinal hemorrhages and radiation papillopathy. OCTA reveals an enlarged foveal avascular zone (FAZ) and capillary dropout in the macular area. The disrupted and reduced capillary network indicates significant macular ischemia. (C) OCT shows thinning of the retina secondary to atrophy. |
Risk Factors
In 1999, a study from Wills Eye Hospital analyzed 1,300 patients with posterior uveal melanoma treated with plaque radiotherapy. They found that 42 percent of patients developed non-proliferative radiation maculopathy at the five-year mark, while 8 percent progressed to proliferative radiation maculopathy.16 After three years, over half of the patients treated with plaque brachytherapy have visual acuity worse than 20/200 (Figure 2).3 What factors influence the risk of developing visually significant RR? The dose of radiation and the location and size of the tumor play a crucial role. Tumors located within 4 mm of the foveola, or 5 mm of the optic disc are associated with an increased risk of RM.16,17 Larger tumors require more radiation and are associated with increased rates of RR, especially tumors with thickness greater than 4 mm.18
When it comes to external beam radiation, cumulative doses greater than 45 Gy and doses greater than or equal to 1.9 Gy per fraction have been linked to the development of RR.19 However, a technique called hyperfractionation, where the total dose is divided into smaller fractions, can reduce the risk of RR in patients receiving more than a 50 Gy total dose.20
Nevertheless, radiation isn’t the only factor at play. Patients with underlying vascular diseases, such as diabetes or hypertension, are more likely to develop RR.17,21 In fact, for diabetic patients, the risk of visual loss from RR was reported to increase by a staggering 300 percent.22 The synergistic effect of diabetes (affecting the pericytes) and radiation (affecting the endothelial cells) is a dangerous combination for the retinal vasculature. Age is another important consideration. Younger patients, particularly those under 50, are at higher risk of developing RR.17,21 This mirrors what we see in diabetes, where a younger age at presentation is associated with a higher risk of developing proliferative diabetic retinopathy.23 A recent study from Wills Eye Hospital in 2020 looked at 1,131 eyes treated with prophylactic anti-VEGF therapy after plaque radiotherapy and confirmed that patients under 50 were more likely to develop both RM and RR compared to older age groups.24
In 2019, one of this article’s authors (LD) and co-authors developed a nomogram for visual acuity after plaque radiotherapy based upon clinical risk factors.25 They found the most important risk factors were initial presentation of the tumor with subretinal fluid involving four quadrants, tumor thickness >4 mm, presenting visual acuity ≤20/30, non-Caucasian race, tumor shape (mushroom, bilobed or multilobulated) and insulin-dependent diabetes.25 Risk of poor visual acuity at two and four years increased from 11 percent and 24 percent with 40 nomogram points to 97 percent and >99 percent with 304 points.25 These findings underscore the importance of individualized risk assessment and long-term monitoring for patients undergoing radiation therapy for ocular tumors. By understanding the factors that influence the development of RR, we can better counsel our patients, tailor our treatment strategies and work towards preserving vision in the face of this challenging complication.
Management
When it comes to treating RR, our primary goals are to halt the progression of the disease and preserve visual acuity. It’s important to note that currently, there’s no FDA-approved treatment specifically for RR, and the therapies we’ll discuss are considered “off-label” uses. The data in this field are largely derived from clinician-led pilot studies or retrospective chart reviews, and there is a notable scarcity of large-scale randomized controlled trials.
• Laser photocoagulation. Before the era of anti-VEGF therapy, retinal laser therapies were the go-to treatment for RR. The idea was to kill off the ischemic tissues that were releasing VEGF and driving neovascularization. Multiple studies showed promising results, with panretinal photocoagulation or focal laser photocoagulation improving or stabilizing visual acuity and decreasing macular edema in patients with RR.26-29 Panretinal photocoagulation can be especially useful in the setting of proliferative RR to cause lasting regression of neovascularization and prevent recurrent vitreous hemorrhage. However, there are some limitations to laser therapy, particularly when ocular tumors involve the fovea or optic disc. In these cases, the risks of laser treatment may outweigh the potential benefits.
• Anti-VEGF therapy. Intravitreal anti-VEGF therapies have revolutionized the treatment of RR, much like they have for other ischemia-driven retinal pathologies. The mid-2000s marked a turning point in the management of RR, with the introduction of these game-changing medications. A 10-year prospective study from Dr. Paul Finger’s team at New York Eye and Ear’s ocular oncology service treated 120 patients with uveal melanoma who had developed macular vasculopathy after plaque brachytherapy. The results were impressive: Anti-VEGF agents (bevacizumab and ranibizumab) were found to be both safe and effective in reducing macular edema, and 80 percent of patients maintained their visual acuity within two lines or better.6
Subsequently, Timothy Murray, MD, and his team at Bascom Palmer Eye Institute conducted a two-year prospective trial using aflibercept. They included 40 patients with uveal melanoma treated with plaque brachytherapy who had developed visually compromising radiation maculopathy. The results mirrored those of Dr. Finger’s study: Aflibercept was deemed safe and effective for maintaining VA and reducing macular edema.30
Furthermore, aflibercept may be more effective as a second-line treatment in patients who’ve failed bevacizumab treatment for RR. A study in 2022 looked at 30 patients who had previously failed bevacizumab treatment. When switched to aflibercept, the patients showed both statistical and clinical improvement in VA and central foveal thickness.31 The Aflibercept for Radiation Retinopathy Trial (ARRT) from Retinal Consultants of Texas took a slightly different approach. They conducted a small, randomized trial of 30 patients with clinical evidence of macular edema after radiation. The focus of the study was to investigate a “treat-and-extend” regimen of aflibercept injections.32 The results were promising—96.7 percent of the entire cohort had VA better than 20/200 at one year, and the treat-and-extend group showed significant improvement in central retinal thickness (182.4 µm) and VA (6.69 letters).32
Another Phase IIb randomized trial, from the Ranibizumab for Radiation Retinopathy (RRR) Study Group, investigated the efficacy of a treat-and-extend ranibizumab regimen in 40 eyes with radiation-induced macular edema and decreased visual acuity.33 During the first year, the cohort received monthly ranibizumab injections, which resulted in statistically significant improvements in visual outcomes, with a gain of four letters, and a reduction in central macular thickness.33 In the second year, the study protocol was modified to treat-and-extend ranibizumab to more closely mimic real-world clinical care.33 At the end of the two-year study period, some of the initial visual improvements had regressed, with a loss of 1.9 letters compared to the end of year one. However, visual acuity remained above baseline levels, and the anatomic improvements in central macular thickness were maintained, with no significant change from baseline at the study’s conclusion.33
Brolucizumab, a relatively new anti-VEGF agent, is currently undergoing testing for macular edema, including in the setting of RR patients who have failed prior anti-VEGF treatments. While there have been some concerns about intraocular inflammation and vasculitis with brolucizumab, it may still hold promise for complex eyes that have been unresponsive to aggressive intravitreal pharmacotherapy.34
To sum it all up, a recent systematic review and meta-analysis of seven studies, including a total of 922 patients, found that anti-VEGF therapy can significantly improve VA (-0.34 logMAR) and reduce central retinal thickness (-34.65 µm).35 However, it’s important to note that improving macular edema, as measured by OCT, doesn’t always translate to improved visual outcomes, especially in eyes that have undergone ischemic changes or repeated periods of oxidative stress.
• Steroids. Corticosteroids have also been explored as a potential treatment option for RR, given their anti-inflammatory properties. The rationale behind their use is that radiation induces an inflammatory reaction in the retina, and steroids may help to mitigate this response. Several small studies have investigated the use of intravitreal dexamethasone or fluocinolone acetonide implants in the treatment of RR. A team from the University of Padova in Italy looked at 13 patients treated with intravitreal dexamethasone, while a German study included five patients who received fluocinolone acetonide implants. Both studies reported improvements in macular edema and VA.36,37
Arun Singh, MD, and colleagues from the Cleveland Clinic Cole Eye Institute took a slightly different approach. They trialed “rescue” steroids in seven patients who had previously failed anti-VEGF treatment. These patients received fluocinolone acetonide implants, and the results showed stable VA and a reduction in central retinal thickness (-30 µm).38 One of the potential advantages of steroid implants is that they reduce the burden of frequent intravitreal injections, which can improve patient satisfaction. However, the superiority of steroids over anti-VEGF therapy in the treatment of RR remains unclear. A study by Berlin’s Ira Siebel and his colleagues in 2016 compared intravitreal anti-VEGF (n=38) with intravitreal steroids (n=35) and found no significant differences between the two treatment modalities.39 It’s important to approach the use of steroids cautiously in patients who have undergone radiation treatment. Steroids have the potential to exacerbate glaucoma or contribute to the development of radiation-induced cataract.
• Prophylaxis. The concept of prophylactic treatment for RR has gained significant attention in recent years, fueled by the advent of advanced imaging techniques like OCTA that allow for earlier detection of retinal vascular changes. The rationale behind this approach is to intervene before visual or clinical signs of RR develop, with the goal of preserving vision and preventing the progression of retinal damage.
One of the initial studies that sparked interest in prophylactic treatment was the 10-year anti-VEGF study by Dr. Finger and associates. While they found that anti-VEGF therapy improved the clinical manifestations of radiation maculopathy, the patients still developed evidence of retinal microaneurysms, capillary nonperfusion and retinal telangiectasias.6 This suggested that despite ongoing periodic anti-VEGF treatment, the disease process was progressive and required more intensive treatment over time.6
Drawing parallels to other progressive retinal vasculopathies, like diabetic retinopathy, where earlier intervention is associated with better outcomes, researchers began to investigate the potential benefits of prophylactic management of RR.2 A study from Wills Eye Hospital, led by Carol Shields, MD, and associates, compared prophylactic bevacizumab (given every four months starting after plaque removal) with a historical control group who didn’t receive bevacizumab.40 This large 10-year retrospective study included 1,131 eyes and demonstrated that the treatment group had better visual acuity outcomes than the controls every year for four years.40 There was also significantly less clinical evidence of RM at 24, 36 and 48 months, and less macular edema at 24 and 36 months.40
In the setting of proton beam irradiation for uveal melanoma, a pilot study from Mass Eye and Ear, led by Ivana Kim, MD, investigated prophylactic ranibizumab (given every two months for two years) in 40 patients. They found that 97 percent (30/31) of the prophylactic ranibizumab group had VA better than 20/200, compared to 42 percent (92/105) of controls.42 This effect was more pronounced in small/medium tumors, likely due to the lower dose of radiation, with only a third (8/24) developing clinical evidence of radiation maculopathy versus 68 percent (42/62) of controls.42 Tara McCannel, MD, PhD, from UCLA reported that vitrectomy with silicone oil tamponade in conjunction with plaque brachytherapy could be useful in preventing RR. This prophylactic silicone oil placement was found to reduce central macular thickness and improve VA in 20 patients undergoing treatment for uveal melanoma.43
Other treatment strategies have been proposed to reduce the risk of radiation retinopathy, although their efficacy has yet to be reported. Image-guided radiation, a real-time imaging technique used to deliver external beam radiation directly to the tumor without damaging the surrounding healthy retina, is one such approach.44 Dose hyperfractionation is another method.20
Future Directions
The upcoming randomized clinical trial, Protocol AL, led by Arun Singh, MD, in the Diabetic Retinopathy Clinical Research Retina Network, is an exciting development in the field of RR research.45 This study will evaluate how prophylactic, repeated injections of faricimab or fluocinolone acetonide implants affect long-term vision compared to initial observation and as-needed treatment for radiation side effects, with the goal of determining if these interventions can prevent or alter the course of macular edema and visual acuity outcomes related to RR.45
Another important aspect of this study is the evaluation of the natural history of RR using multimodal imaging techniques, such as fundus photography, FA and OCTA.45 This comprehensive approach to monitoring the progression of RR will provide valuable insights into the disease process and may help to identify early markers of retinal damage that could guide future treatment strategies. The significance of this trial can’t be overstated, as RR is a devastating consequence of radiation therapy that can have a profound impact on patients’ quality of life.
As we have discussed throughout this review, RR is a common and visually significant complication, and its risks should be thoroughly discussed with patients undergoing radiation for ocular, head or neck malignancies.
The varied clinical presentations of RR highlight the importance of maintaining a high index of suspicion in any patient with a history of radiation exposure. Clinicians must be vigilant in monitoring for signs of RR, even years after the initial treatment, and should consider multimodal imaging techniques to detect subtle changes in retinal vasculature before visual decline or overt clinical signs manifest.
Emerging evidence for the prevention of RR, particularly with the use of prophylactic anti-VEGF therapy, is promising and offers hope for delaying or reducing the incidence and severity of this condition. However, current management strategies still primarily aim to prevent progression and further visual loss once RR has developed.
Treatment options for RR are varied and often mirror the treatments available for other common vasculopathies, such as diabetic retinopathy. These include anti-VEGF agents, corticosteroids and laser photocoagulation. However, it’s important to acknowledge that the evidence for these treatments in the specific context of RR is limited, with most studies being small and retrospective in nature.
In conclusion, RR remains a significant challenge for patients undergoing radiation therapy for certain malignancies. The upcoming DRCR Retina Network trial, Protocol AL, represents an important step forward in our understanding of this condition and the potential for early intervention to prevent or mitigate vision loss.
In the years to come, we’ll have a stronger evidence basis for RR management decisions as a result of this trial. In the meantime, as clinicians, it’s our responsibility to remain vigilant in monitoring for signs of RR, to use the latest imaging techniques to detect early changes in retinal vasculature, and to provide our patients with the best available evidence-based care.
While there’s still much work to be done in this field, the future holds promise for improving outcomes and preserving vision in patients at risk for radiation retinopathy.
This publication was supported by CTSA Grant Number KL2 TR002379 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Ms. Krivit is a medical student at the University of Queensland, Australia. Dr. Yaghy is a first-year resident at the Department of Ophthalmology and Visual Sciences, University of Massachusetts Chan Medical School in Worcester. Dr. Dalvin is an associate professor of ophthalmology and a senior associate consultant in the Division of Medical Oncology, Department of Oncology at the Mayo Clinic in Rochester. She is a Consultant for Ideaya Biosciences. Neither of the other authors have a financial interest in any of the material presented.
Correspondence to: Lauren A. Dalvin, MD.
Email address: dalvin.lauren@mayo.edu
1.Ramos MS, Echegaray JJ, Kuhn-Asif S, Wilkinson A, Yuan A, Singh AD, et al. Animal models of radiation retinopathy—From teletherapy to brachytherapy. Exp Eye Res 2019;181:240-51.
2.Powell BE, Chin KJ, Finger PT. Early anti-VEGF treatment for radiation maculopathy and optic neuropathy: Lessons learned. Eye (Lond) 2023;37:5:866-74.
3.Diener-West M, Earle JD, Fine SL, Hawkins BS, Moy CS, Reynolds SM, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS Report No. 18. Arch Ophthalmol 2001;119:7:969-82.
4.Zamber RW, Kinyoun JL. Radiation retinopathy. West J Med 1992;157:5:530-3.
5.Chen K, Browne AW. Radiation Retinopathy. Current Ophthalmology Reports 2023;11:3:49-56.
6.Finger PT, Chin KJ, Semenova EA. Intravitreal anti-VEGF therapy for macular radiation retinopathy: a 10-year study. Eur J Ophthalmol 2016;26:1:60-6.
7.Bianciotto C, Shields CL, Pirondini C, Mashayekhi A, Furuta M, Shields JA. Proliferative radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology 2010;117:5:1005-12.
8.Sahoo NK, Ranjan R, Tyagi M, Agrawal H, Reddy S. Radiation retinopathy: detection and management strategies. Clin Ophthalmol. 2021;15:3797-809.
9.Brown GC, Shields JA, Sanborn G, Augsburger JJ, Savino PJ, Schatz NJ. Radiation retinopathy. Ophthalmology 1982;89:12:1494-501.
10.Amoaku WM, Archer DB. Fluorescein angiographic features, natural course and treatment of radiation retinopathy. Eye (Lond) 1990;4(Pt 5):657-67.
11. Horgan N, Shields CL, Mashayekhi A, Teixeira LF, Materin MA, Shields JA. Early macular morphological changes following plaque radiotherapy for uveal melanoma. Retina 2008;28:2:263-73.
12. Shields CL, Say EA, Samara WA, Khoo CT, Mashayekhi A, Shields JA. Optical coherence tomography angiography of the macula after plaque radiotherapy of choroidal melanoma: Comparison of irradiated versus nonirradiated eyes in 65 patients. Retina 2016;36:8:1493-505.
13. Matet A, Daruich A, Zografos L. Radiation maculopathy after proton beam therapy for uveal melanoma: Optical coherence tomography angiography alterations influencing visual acuity. Invest Ophthalmol Vis Sci 2017;58:10:3851-61.
14.Skalet AH, Liu L, Binder C, Miller AK, Crilly R, Hung AY, et al. Longitudinal detection of radiation-induced peripapillary and macular retinal capillary ischemia using OCT angiography. Ophthalmol Retina 2020;4:3:320-6.
15.Skalet AH, Liu L, Binder C, Miller AK, Wang J, Wilson DJ, et al. Quantitative OCT angiography evaluation of peripapillary retinal circulation after plaque brachytherapy. Ophthalmol Retina 2018;2:3:244-50.
16.Gündüz K, Shields CL, Shields JA, Cater J, Freire JE, Brady LW. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol 1999;117:5:609-14.
17.Krema H, Xu W, Payne D, Vasquez LM, Pavlin CJ, Simpson R. Factors predictive of radiation retinopathy post (125)Iodine brachytherapy for uveal melanoma. Can J Ophthalmol 2011;46:2:158-63.
18.Stack R, Elder M, Abdelaal A, Hidajat R, Clemett R. New Zealand experience of I125 brachytherapy for choroidal melanoma. Clin Exp Ophthalmol 2005;33:5:490-4.
19. Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: Analysis of time-dose factors. Int J Radiat Oncol Biol Phys 1994;30:4:765-73.
20. Monroe AT, Bhandare N, Morris CG, Mendenhall WM. Preventing radiation retinopathy with hyperfractionation. Int J Radiat Oncol Biol Phys 2005;61:3:856-64.
21. Gragoudas ES, Li W, Lane AM, Munzenrider J, Egan KM. Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology 1999;106:8:1571-7; discussion 7-8.
22. Viebahn M, Barricks ME, Osterloh MD. Synergism between diabetic and radiation retinopathy: Case report and review. Br J Ophthalmol 1991;75:10:629-32.
23. Hietala K, Harjutsalo V, Forsblom C, Summanen P, Groop PH. Age at onset and the risk of proliferative retinopathy in type 1 diabetes. Diabetes Care 2010;33:6:1315-9.
24. Chang M, Dalvin LA, Mazloumi M, Martin A, Yaghy A, Yang X, et al. Prophylactic intravitreal bevacizumab after plaque radiotherapy for uveal melanoma: Analysis of visual acuity, tumor response, and radiation complications in 1131 eyes based on patient age. Asia Pac J Ophthalmol (Phila) 2020;9:1:29-38.
25. Dalvin LA, Zhang Q, Hamershock RA, Chang M, Yu MD, Mashayekhi A, et al. Nomogram for visual acuity outcome after iodine-125 plaque radiotherapy and prophylactic intravitreal bevacizumab for uveal melanoma in 1131 patients. Br J Ophthalmol 2020;104:5:697-702.
26. Finger PT, Kurli M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Br J Ophthalmol 2005;896:730-8.
27. Hykin PG, Shields CL, Shields JA, Arevalo JF. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology 1998;1058:1425-9.
28. Kinyoun JL. Long-term visual acuity results of treated and untreated radiation retinopathy (an AOS thesis). Trans Am Ophthalmol Soc 2008;106:325-35.
29. Kinyoun JL, Lawrence BS, Barlow WE. Proliferative radiation retinopathy. Arch Ophthalmol 1996;1149:1097-100.
30. Murray TG, Latiff A, Villegas VM, Gold AS. Aflibercept for radiation maculopathy (ARM Study): Year-2 extension of a prospective clinical study. J Vitreoretin Dis 2021;53:232-8.
31. Srivastava O, Weis E. Outcomes of second-line intravitreal anti-VEGF switch therapy in radiation retinopathy secondary to uveal melanoma: Moving from bevacizumab to aflibercept. Ocul Oncol Pathol 2023:84-6:230-5.
32. Trejo Corona S, Villanueva Boone C, Ali AM, Moore C, Brown A, Munoz J, et al. Randomized trial of treat-and-extend intravitreal aflibercept for radiation retinopathy: 1-Year outcomes. Invest Ophthalmol Vis Sci 2023;647:35.
33. Yu HJ, Fuller D, Anand R, Fuller T, Munoz J, Moore C, et al. Two-year results for ranibizumab for radiation retinopathy (RRR): A randomized, prospective trial. Graefes Arch Clin Exp Ophthalmol 2022;2601:47-54.
34. Murray JE, Gold AS, Latiff A, Murray TG. Brolucizumab: Evaluation of compassionate use of a complex anti-VEGF Therapy. Clin Ophthalmol 2021;15:4731-8.
35. Zhuang J, Yang Y, Liao Y, Li C, Wang WA, Luo X, et al. Efficacy of intravitreal injections anti-vascular endothelial growth factor treatment for radiation retinopathy: A systematic review and meta-analysis. Am J Ophthalmol 2024;263:141-51.
36. Frizziero L, Parrozzani R, Trainiti S, Pilotto E, Miglionico G, Pulze S, et al. Intravitreal dexamethasone implant in radiation-induced macular oedema. Br J Ophthalmol 2017;10112:1699-703.
37. Zimmermann L, Kneifel C, Grajewski L, Ciernik IF, Krause L. Treatment of radiation-induced maculopathy with fluocinolone acetonide. Graefe’s Archive for Clinical and Experimental Ophthalmology 2020;25811:2535-9.
38. Singaravelu J, Oakey ZB, Wrenn JM, Singh AD. Intravitreal fluocinolone acetonide implant for radiation retinopathy: Report of preliminary findings. Retina 2023;438:1364-9.
39. Seibel I, Riechardt AI, Davids A-M, Böker A, Rehak M, Hager A, et al. Radiation retinopathy after proton beam therapy in uveal melanoma. Investigative Ophthalmology & Visual Science 2016;5712:4097.
40. Shields CL, Dalvin LA, Chang M, Mazloumi M, Fortin P, McGarrey M, et al. Visual outcome at 4 years following plaque radiotherapy and prophylactic intravitreal bevacizumab (every 4 months for 2 years) for uveal melanoma: Comparison with nonrandomized historical control individuals. JAMA Ophthalmol 2020;1382:136-46.
41. Powell BE, Finger PT. Anti-VEGF Therapy immediately after plaque radiation therapy prevents or delays radiation maculopathy. Ophthalmol Retina 2020;45:547-50.
42. Kim IK, Lane AM, Jain P, Awh C, Gragoudas ES. Ranibizumab for the prevention of radiation complications in patients treated with proton beam irradiation for choroidal melanoma. Trans Am Ophthalmol Soc 2016;114:T2.
43. McCannel TA, McCannel CA. Iodine 125 brachytherapy with vitrectomy and silicone oil in the treatment of uveal melanoma: 1-to-1 matched case-control series. Int J Radiat Oncol Biol Phys 2014;892:347-52.
44. Eibl-Lindner K, Fürweger C, Nentwich M, Foerster P, Wowra B, Schaller U, Muacevic A. Robotic radiosurgery for the treatment of medium and large uveal melanoma. Melanoma Res 2016;26:1:51-7.
45. Intravitreal Faricimab injections or fluocinolone acetonide (0.19 mg) intravitreal implants vs observation for prevention of VA loss due to radiation retinopathy (AL): ClinicalTrials.gov. 2023 [updated May 6, 2024. NCT05844982]. Available from: https://clinicaltrials.gov/study/NCT05844982?cond=Radiation%20Retinopathy&aggFilters=status:rec&rank=1.



