They say that time heals all, but perhaps a more accurate argument would be that time gives us the data to heal all. Twenty years ago, patients diagnosed with age-related macular degeneration would have few treatment options. With AREDS studies just taking off, treatment for neovascular AMD would have been limited to photodynamic therapy or laser photocoagulation. Over time, new players entered the game, quickly changing the paradigm of the field of retina and our understanding of AMD. Success in clinical trials helped anti-VEGF drugs such as bevacizumab, ranibizumab and aflibercept become the mainstays of neovascular AMD treatment.1-3 Today, we even have options for patients with advanced dry AMD, with pegcetacoplan and avacincaptad pegol entering the market following positive trial results.4 However, current treatments require adherence to strict treatment windows, are associated with potential complications such as endophthalmitis and retinal vasculitis, and don’t have the potential to restore the cellular damage already caused by the disease. This is the gap that regenerative therapies aim to fill.
Cell therapy, if proven to be a viable form of treatment, could help alleviate some of the limitations associated with our current standard of care in ophthalmology. In this article, we’ll describe the pipeline for stem cell trials hoping to make some headway not only for AMD, but also for retinal dystrophies, including retinitis pigmentosa and Stargardt disease. Unlike gene replacement therapy where specific genes are replaced for specific genetic conditions, cell therapy is disease-agnostic. This means that one cell therapy may be able to provide treatment for multiple diseases, regardless of genetic makeup or even disease entity.
Astellas/ACT Trials for Stargardt Macular Dystrophy
Stargardt macular dystrophy (SMD) is the most common form of juvenile macular dystrophy and has no current FDA-approved treatments on the market. It may be associated with mutations of the ABCA4 gene, causing dysregulation of the photoreceptor retinoid cycle ultimately leading to gradual RPE and photoreceptor cell death.5 Eyes with advanced SMD often have limited vision due to macular atrophy.
Embryonic stem cells, given their ability to differentiate into various cell types, have theoretical potential to repair and replace degenerated tissues.6 In 2010, the FDA approved the second ever U.S. clinical trial to test human embryonic stem cells (hESCs), and the first to use them in patients with SMD.7 Now acquired by Astellas Pharma, the 7316-CL-0001 study was initiated by Advanced Cell Technology (ACT) as a Phase I/II safety and tolerability assessment for the use of hESC-derived RPE cells (named ASP7316 or MA09-hRPE) in patients with SMD.8
An identical design was used in simultaneous U.K.-based SMD trials, under the protocol 7316-CL-0003.9 The U.S. study enrolled 13 patients, divided into four “worse vision” cohorts and one “better vision” cohort based on visual acuity at presentation. Each cohort was assigned a dose ranging from 50,000 to 200,000 hESC RPE cells, with the same design replicated for the U.K. protocol, 7316-CL-0003, in which a total of 12 patients were enrolled. Each patient underwent a pars plana vitrectomy in the worse-seeing eye, followed by subretinal injection of viable hESC-RPEs in suspension form. A regimen of tacrolimus and mycophenolate mofetil was introduced a few days before surgery to prevent rejection. Patients were followed over the course of 52 weeks following cell transplant in the initial U.S. and U.K. protocols, and all continued onto respective five-year long-term follow-up studies.
At six months follow-up in the U.S. trial, three patients had a gain of ≥15 ETDRS best-corrected visual acuity letters, four had maintenance of baseline acuity, and one patient had a decrease of ≥10 letters.10 At one year, four patients experienced an improvement of ≥10 letters while no fellow untreated eyes experienced a gain of ≥10 letters. Overall, transplantation was successful without failure or rejection in all 13 patients through the one year follow-up period. The most prevalent postoperative complication was significant cataract progression, as seen in three out of 13 patients in the U.S. trial (two of which required cataract surgery). Two cases of vitreous inflammation were also noted in this trial: One was attributed to endophthalmitis, and the other remained stable until spontaneous resolution by month six.10,11
Data from the U.S. LTFU study, protocol 7317-CL-0004, showed that the treatment was safe and well-tolerated at all doses, with no major safety events supporting dose-dependent trends. No immune responses or adverse events pointing to cell graft rejection or failure were noted, and visual acuity data demonstrated a sharper decline in the BCVA letter score for untreated eyes as opposed to treated eyes through the end of the study. At one year post-transplant, eyes treated on the initial study had an average BCVA letter decline of 6.7 letters, compared to a mean decrease of 1.3 letters in the untreated eyes. However, at five years post-transplant, treated eyes experienced an average BCVA letter score decrease of eight letters, compared to 20.7 in eyes that weren’t treated.12, 13
LTFU data from U.K. protocol 7316-CL-0006 similarly reported no evidence of graft failure or rejection in any of the patients. Notably, there was also no remarkable difference in BCVA between treated and untreated eyes. Among the 12 patients enrolled, the mean change in BCVA letter score for treated eyes between the baseline visit and the month 60 post-transplant assessment was 2.8 letters, compared to an average change of two letters in untreated eyes. Epiretinal membrane formation was observed more frequently in treated eyes, although the number of cases seen for each hasn’t yet been published.14,15 While data regarding the rate of retinal detachments has yet to be disclosed for these trials, it should be noted that this is an additional risk factor to be considered with many invasive transvitreal procedures including PPVs and delivery of subretinal therapies.
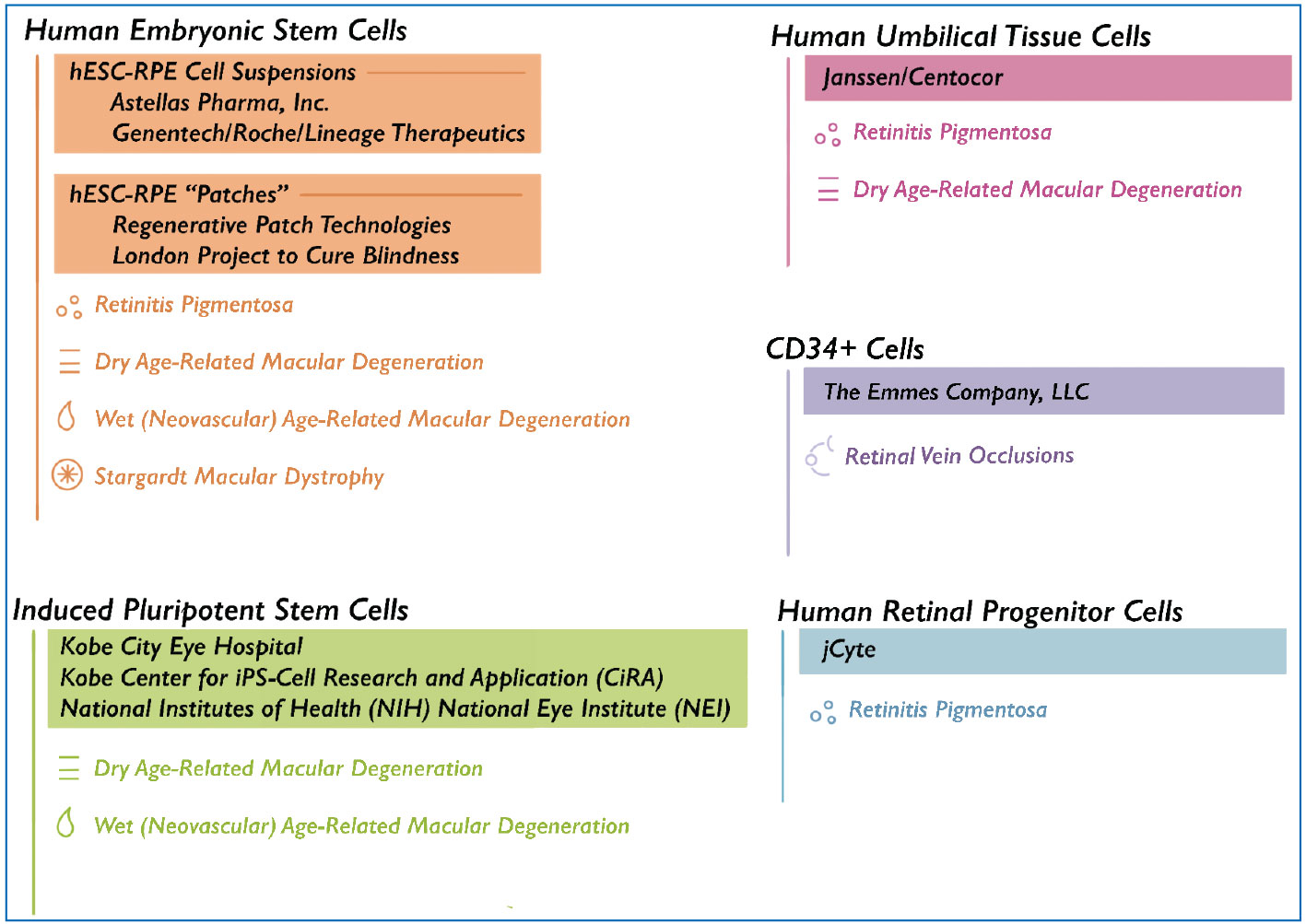 |
|
A list of the companies investigating cell therapies for various retinal diseases. |
Astellas Trials for GA Secondary to AMD
Photoreceptor and RPE degeneration with central vision loss is the hallmark of geographic atrophy in AMD. In the early 2010s, a cell therapy protocol for this subset of patients was initiated to determine whether the Astellas ASP7316 line of hESC-RPEs could be a safe and tolerable treatment consideration for the future. The ASP7316 cell line’s Phase I/II safety and tolerability trial for U.S. patients with advanced dry age-related macular degeneration was conducted under the protocol 7316-CL-0002.16 A total of 13 patients were treated in this study, which followed a dose-escalation design starting at a 50,000-cell transplant, increasing in sequences of 50,000 cells-per-cohort until a maximum of 200,000 cells was reached. As with the SMD studies, the cells were transplanted in the subretinal space following PPV in the eye presenting with worse vision. Upon completion of the initial trial, 11 subjects were enrolled into the corresponding five-year LTFU protocol, 7316-CL-0005, for further review.17
 |
|
Common retinal diseases studied for the use of cell therapy. |
Although data analysis of the follow-up study remains unpublished, a summary report released by Astellas stated that it was overall well-tolerated, with no signs of graft failure, immune response or rejection. As with the initial SMD studies, data indicating the frequency of common postoperative complications such as RDs and ERM formation have yet to be published, but should still be noted as a potential risk for any patient. Vision data demonstrated that between months 12 and 24 postop, the average BCVA score increased by 10.4 letters in the treated eye, and 3.8 letters in the untreated eye. However, by month 30 post-transplant BCVA letter scores decreased by 1.6 and 0.7 letters in the treated and untreated eyes, respectively.18 A follow-up report analyzed these BCVA trends and reported better averages for subjects that didn’t experience postoperative cataract formation.10
Given the success in safety and tolerability of these previous trials, the Astellas pipeline now includes an updated product of hESC-RPE cells (ASP7317), which will continue to be investigated as a potential treatment for GA and SMD. The Phase Ib 7317-CL-0003 trial is actively enrolling, gathering safety and tolerability data, as well as efficacy results related to potential impacts to vision and GA lesion size.19 The trial is expected to conclude in late 2025, with its design following that of the earlier, dose-escalation method of treating patients with 50,000 to 200,000 cells. As with previous Astellas protocols, this surgical procedure includes a PPV, followed by subretinal injection of the cell dosage designated to each patient based on cohort selection.
Roche/Genentech (Previously CellCure/Lineage) Trials for GA
In 2015, Lineage Cell Therapeutics began a Phase I/IIa dose-escalation trial testing a cell suspension of directly differentiated hESC-RPE cells (RG6501) in patients with GA in both eyes secondary to dry AMD. Similar to the cell therapy used throughout the Astellas pipeline, the Lineage trial investigated hESC-RPE cells for safety and tolerability delivered in two forms. The first form was a standard subretinal implantation following PPV, and the second consisted of an FDA-approved suprachoroidal to subretinal delivery (Orbit Subretinal Delivery System) of the cells, performed in seven out of 24 enrolled patients. A total of 12 patients were treated with RG6501 doses between 50,000 and 150,000 cells, while the remaining 12 patients received 200,000 cell units.20,21
The first phase of the trial followed subjects until a year post-transplant, and 21 subjects have continued into the second phase: A period of routine observation lasting up to five years. This study is still actively collecting data as part of its follow-up segment. Endpoints include safety and tolerability analyses as well as changes to the rate of GA progression. Subjects were assigned tacrolimus and mycophenolate therapy for six and 12 weeks post-transplant, respectively.22
Overall, data demonstrated that the hESC-RPEs were well-tolerated in most patients, although two of 17 patients in the PPV group experienced RDs post-transplant, and 15 out of 17 patients in the group experienced mild to moderate ERM formation. Both retinal detachments observed were subsequently treated and resolved, and out of the 15 ERM cases, three required surgical peeling. Among the seven patients treated with RG6501 via suprachoroidal delivery, choroidal neovascularization was noted in three cases, one of which resolved completely following a single treatment with anti-VEGF. The remaining two CNV patients required regular anti-VEGF administration following AE onset.21
In terms of visual changes and disease progression over time, lower-dose cohorts followed a relatively typical course of GA progression from surgery to long-term follow-up, with no remarkable results being disclosed in interim results.
The 200,000-cell group, however, showed more promising results, particularly in the smaller GA lesion and better baseline VA cohort and when the subretinal bleb showed extensive coverage of the GA lesion. Out of 12 patients enrolled in that cohort, three (25 percent) had a BCVA improvement ≥15 letters in the treated eye at year one post-transplant. The average BCVA change by year one post-transplant was a gain of 7.6 letters. Untreated eyes, however, declined in BCVA letter score on average.
Similarly, fundus autofluorescence imaging showed that treated eyes across all cohorts demonstrated reduced rates of RPE loss at the border of GA lesions.23,24 Subjects with extensive bleb coverage demonstrated improvements in outer retinal structure on qualitative OCT analysis by a masked grader. The Phase I/IIa trial, currently in the observational phase, is expected to conclude in mid-2025.
Another Phase IIa RG6501 GA study from Roche and Genentech, protocol GR44251, is currently active and enrolling close to 60 patients in California, Ohio and Pennsylvania.25,26 Unlike the initial dose-escalating study, this trial is focusing only on safety and efficacy results of the 200,000 hESC-RPE cell dosage, with hopes to provide more statistical power to the data in the Phase I/IIa Lineage trial’s high-dose cohort results.
Regenerative Patch Technologies Trials for GA
A 2016 study conducted by Regenerative Patch Technologies (RPT) explored the use of a bioengineered retinal “patch” implant for GA, consisting of a monolayer of hESC-derived mature RPE cells adhered to a perylene scaffold.27
The cell product (CPCB-RPE1) was delivered subretinally to 16 patients enrolled in this Phase I/IIa trial, completely covering the atrophic lesion in each study eye. Tacrolimus and intravenous steroids were provided to all subjects.
The surgical method for this study was published in 2020 by Amir Kashani, MD, PhD, and colleagues at the University of Southern California Roski Eye Institute.28 Notably, this monolayer implantation required subretinal hydrodissection of the GA lesion, resulting in a longer average surgery time compared to trials using a hESC-RPE suspension. This technique might reduce the percentage of cells that reflux into the vitreous as compared to subretinal cell injections, with RPE cells that are all aligned in the correct orientation. Overall, 86.9 percent of the baseline GA area was covered by the monolayer implant across all study participants.
With 15 out of 16 patients receiving successful implantation of CPCB-RPE1 into the subretinal space, the RPE monolayer delivery method was deemed feasible for patients with GA.28 Retinal hemorrhages were commonly noted throughout the study but were mostly transient following the surgical procedure. Over three years of postoperative follow-up, treated eyes were more likely to experience a >5 letter BCVA increase than untreated eyes—which had a higher likelihood of decreasing by >5 BCVA letters.29 A Phase IIb trial is expected next in the pipeline, to provide more data on safety and potential efficacy of the bioengineered implant.
Janssen/Centocor Trials for Retinitis Pigmentosa and AMD
Cell therapy research has a broader scope beyond hESCs. Janssen/Centocor trials have attempted to determine the efficacy and immunogenicity of human umbilical tissue-derived RPE cells (hUTC-RPEs; named palucorcel, or CNTO 2476) in degenerative retinal diseases.
Unlike hESCs, these cells are derived from adult stem cells which are multipotent, meaning that they’re able to differentiate into different cell types that can promote repair to damaged tissues.30 First clinically tested in 2007, Centocor’s Phase I CNTO 2476 trial was geared towards patients with retinitis pigmentosa, a group of genetic disorders causing photoreceptor degeneration and gradual vision loss.31 After seven patients were enrolled and treated, the trial was terminated early in 2013 due to the development of RDs in two subjects. This RD rate was mostly attributed to the surgical delivery method used in the trial, which was transvitreal delivery of the cell line into the subretinal space. The treatment itself (CNTO 2476) was considered “possibly related” to the RDs. In addition, this hUTC trial didn’t use immunosuppressive therapies, and a closer histological study of an epiretinal membrane sampled from one of the RD cases on the trial was unable to confirm the immunological safety of CNTO 2476.32
Once acquired by Janssen Research & Development, a Phase I/IIa study was initiated in 2010 to test the safety, tolerability and efficacy of the CNTO 2476 line in GA patients.
CNTO 2476 was administered in 33 patients enrolled in the CR017548 trial, and while there were no observed cases of immune response or rejection, the high rate of RDs and retinal tears noted (17.1 and 37.1 percent, respectively) emphasized the need for further modifications to the surgical approach. Nevertheless, visual gain data for eyes treated in this study was optimistic, with 24.1 percent of eyes sustaining a ≥15-letter gain at one year postoperatively, and 34.5 percent showing an improvement of ≥10 letters in the same timeframe.33
The Janssen PRELUDE study followed in 2015, and was conducted with the hope that a suprachoroidal to subretinal approach would reduce the risk of complications noted in earlier trials.34
In this Phase IIb trial, 21 patients received suprachoroidal to subretinal delivery of approximately 300,000 hUTC-RPEs through an updated surgical approach using a custom delivery system for the cell suspension. While adverse events with the new surgical approach were less extreme and the utility of the custom delivery system proved to be successful, data regarding vision and GA progression was confounding, and pointed towards no apparent benefit of the hUTC-RPE treatment.35 The delivery system evolved into the FDA-approved Orbit Subretinal Delivery System.
Kobe Trials of iPSC-RPE Sheets And Retinal Organoids
In addition to hUTCs, the safety and efficacy of induced pluripotent stem cell (iPSC) treatments for retinal diseases has also been an area of major interest. In 2017, a study from the Kobe Center for iPS Cell Research and Application (CiRA) in Japan assessed the feasibility of autologous iPSC-derived RPE cells in two patients with wet AMD.36
As opposed to allogeneic cells such as hESCs, autologous cell therapy derives valuable, differentiable stem cells from the patients themselves, which reduces the risk of transplant rejection, and provides an easier cell harvesting method.37
Two patients were enrolled in this study, where iPSCs were made from their own skin fibroblasts, then successfully prepared into RPE sheets ex vivo. Only one patient was treated with the autologous iPSC-RPE sheet, due to gene deletions detected in the second patient that posed unforeseen risks. For the treated patient, removal of the neovascular membrane was performed prior to subretinal transplantation of the RPE sheet. No signs of graft rejection, failure or any serious adverse events were noted at one year post-transplant, and the presence of RPE cells appeared to grow over time on OCT images. Retinal sensitivity and visual acuity remained stable in the treated eye, at 20/200 throughout the follow-up study period.
Recently, the Kobe City Eye Hospital group published a report following the first clinical trial using iPSC-derived retinal organoid sheets.38 As described, retinal organoids may have more promise than stem cell suspensions or “patches” due to their ability to account for more than just RPE loss in retinal degenerative diseases.
Early animal models demonstrate potential for these organoids to help restore further visual function due to their ability to differentiate into viable photoreceptors, accounting for the deterioration of photoreceptors that occurs in advanced disease.
Subretinal implantation of three organoid sheets was performed in two patients with advanced RP. Intentional shallow retinal detachments were induced in the selected eye, and implantation was localized at sites displaying RPE retention to maximize opportunity for synaptic connections to the host retina. Silicone oil was used as tamponade, while intravitreal triamcinolone and oral cyclosporine were provided to suppress potential inflammation.
There were no reports of intraocular inflammation, graft rejection, or serious systemic adverse events noted through two years of follow-up. While exploratory endpoints demonstrated no significant change to visual functioning outcomes in either patients, the treatment was well-tolerated in both cases.
The Future of Cell Research
Research into cell therapy continues to broaden the scope of its use in ophthalmology and beyond. In 2018, a Phase I trial researching a patch of hESC-RPEs provided hopeful results after treating two patients with severe wet AMD.39 A single-center study in California is applying these new technologies to retinal vein occlusions, testing the safety and viability of CD34+ stem cells in a Phase I/II trial, enrolling 16 patients following Phase I data.40,41
Human retinal progenitor cells (hRPCs) are also being more closely studied for their potential to reactivate photoreceptors in retinitis pigmentosa patients. Recent results of a Phase II safety trial by jCyte demonstrated the safety of intravitreal hRPC injections for RP in 30 patients, with a larger Phase II/III trial anticipated to follow in late 2024.42 Entities such as Astellas and the NIH also plan on continuing the pipeline of iPSCs for retinal disorders due to their efficiency, scalability and potential for treatment.43,44
In conclusion, there is vast potential for cell therapies as a treatment option for a myriad of retinal diseases and degenerations. Although cell therapy retina trials to date have been small, promising safety and tolerability data has emerged even at long term intervals. Current cell therapy trials continue to build on previous studies and we expect this quickly growing field of research to offer us insight into how our treatment approaches may change in the near future.
Ms. Nayar was the lead clinical research coordinator in the Wills Eye Hospital/Mid Atlantic Retina department of Retina Research at the time of writing this article. She is now a clinical research associate.
Dr. Acaba-Berrocal is a PGY4 resident at the Illinois Eye and Ear Infirmary, University of Illinois-Chicago.
Dr. Yonekawa is an associate professor of ophthalmology at Wills Eye Hospital/Mid Atlantic Retina/Thomas Jefferson University. He is an adult and pediatric vitreoretinal surgeon.
Dr. Ho is a professor of ophthalmology at Thomas Jefferson University. He is an attending vitreoretinal surgeon and the director of Retina Research at Wills Eye Hospital/Mid Atlantic Retina in Philadelphia.
1. Patel PJ, Bunce C, Tufail A; ABC Trial Investigators. A randomised, double-masked phase III/IV study of the efficacy and safety of Avastin (bevacizumab) intravitreal injections compared to standard therapy in subjects with choroidal neovascularisation secondary to age-related macular degeneration: Clinical trial design. Trials 2008;9:56.
2. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration [published correction appears in Ophthalmology 2013;120:1:209-10]. Ophthalmology 2012;119:12:2537-2548.
3. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. The New England Journal of Medicine 2006;355:1419-1431.
4. Exploring The Geographic Atrophy Landscape. https://eyesoneyecare.com/resources/exploring-geographic-atrophy-landscape/. [Accessed February 1, 2024].
5. Kohli P, Tripathy K, Kaur K. Stargardt Disease. [Updated 2024 Jan 8]. In: StatPearls [online publication]. Treasure Island, FL: StatPearls Publishing; 2024. Available from: https://www.ncbi.nlm.nih.gov/books/NBK587351/.
6. Lanza R, Gearhart J, Hogan B, et al. Essentials of stem cell biology. 2nd ed. San Diego: Academic Press/Elsevier, 2009.
7. Vogel G. Second trial using human embryonic stem cells gets go-ahead. Science. Nov 22, 2010. doi: 10.1126/article.29614. https://www.science.org/content/article/second-trial-using-human-embryonic-stem-cells-gets-go-ahead. Accessed February 2, 2024.
8. ClinicalTrials.gov. Sub-retinal transplantation of hESC derived RPE(MA09-hRPE)cells in patients with Stargardt’s macular dystrophy. https://clinicaltrials.gov/study/NCT01345006. Accessed February 2, 2024.
9. ClinicalTrials.gov. Safety and tolerability of sub-retinal transplantation of human embryonic stem cell derived retinal pigmented epithelial (hESC-RPE) cells in patients With Stargardt’s macular dystrophy (SMD). https://clinicaltrials.gov/study/NCT01469832. Accessed February 2, 2024.
10. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015;385:9967:509-516.
11. Astellas Institute of Regenerative Medicine. https://s3.amazonaws.com/ctr-ast-7491/7316-CL-0001/6220dd2e-8e29-4488-b7a4-533f2db558d5/b420af0a-afa1-4629-8f43-3edb40e09946/7316-CL-0001_Redacted_CSR_synopsis-v1.pdf. Accessed February 2, 2024.
12. ClinicalTrials.gov. Long term follow up of sub-retinal transplantation of hesc derived rpe cells in Stargardt macular dystrophy patients. https://clinicaltrials.gov/study/NCT02445612. Accessed February 2, 2024.
13. ASP7316, Stargardt’s Macular Dystrophy.https://s3.amazonaws.com/ctr-ast-7491/7316-CL-0004/4d598c84-774c-41fc-bcb4-296e213a54c4/ce07c61f-321d-4ba5-a880-626a453f1bab/7316-cl-0004-clrrs-02-disc01-en-final-03-v1.pdf. Accessed February 2, 2024.
14. ClinicalTrials.gov. A follow up study to determine the safety and tolerability of sub-retinal transplantation of human embryonic stem cell derived retinal pigmented epithelial (hESC-RPE) cells in patients with Stargardt’s macular dystrophy (SMD). https://clinicaltrials.gov/study/NCT02941991. Accessed February 2, 2024.
15. ASP7316, Stargardt’s Macular Dystrophy. https://s3.amazonaws.com/ctr-ast-7491/7316-CL-0006/2c7b0693-42f7-4e74-8af3-d0afd6ab4986/69ee1e60-70a9-4ae7-bd25-c731adb8dff7/7316-cl-0006-clrrs-02-disc01-en-final-02_Redacted-v1.pdf. Accessed February 2, 2024.
16. ClinicalTrials.gov. Safety and tolerability of sub-retinal transplantation of hESC Derived RPE (MA09-hRPE) cells in patients with advanced dry age related macular degeneration (dry AMD). https://clinicaltrials.gov/study/NCT01344993. Accessed February 2, 2024.
17. ClinicalTrials.gov. Long term follow up of sub-retinal transplantation of hESC derived RPE cells in patients with AMD. https://clinicaltrials.gov/study/NCT02463344. Accessed February 2, 2024.
18. ASP7316, Advanced Dry Age-related Macular Degeneration. https://s3.amazonaws.com/ctr-ast-7491/7316-CL-0005/bcdd56e0-b002-4f11-8915-e1172ca9a92b/5920a984-3372-4c28-8d74-42a045853018/7316-cl-0005-clrrs-02-disc01-en-final-03-v1.pdf. Accessed February 2, 2024.
19. ClinicalTrials.gov. A study of the safety and tolerability of ASP7317 in senior adults who are losing their clear, sharp central vision due to geographic atrophy secondary to dry age-related macular degeneration. https://classic.clinicaltrials.gov/ct2/show/NCT03178149. Accessed February 2, 2024.
20. ClinicalTrials.gov. Safety and efficacy study of OpRegen for treatment of advanced dry-form age-related macular degeneration. https://classic.clinicaltrials.gov/ct2/show/NCT02286089. Accessed February 2, 2024.
21. Ho AC, Banin E, Barak A, et al. Safety and efficacy of a phase 1/2a clinical trial of transplanted allogeneic retinal pigmented epithelium (RPE, OpRegen) cells in advanced dry age-related macular degeneration (AMD). Invest Ophthalmol Vis Sci 2022;63;7:1862.
22. ClinicalTrials.gov. https://classic.clinicaltrials.gov/ProvidedDocs/89/NCT02286089/Prot_000.pdf. Accessed February 7, 2024.
23. Banin E, Barak A, Boyer DS, et al. Exploratory optical coherence tomography (OCT) analysis in patients with geographic atrophy (GA) treated by OpRegen: Results from the Phase 1/2a trial. Investigative Ophthalmology & Visual Science;64:2826.
24. Lineage reports fourth case of retinal tissue restoration with OpRegen. https://www.biospace.com/article/releases/lineage-reports-fourth-case-of-retinal-tissue-restoration-with-opregen-/. Accessed February 7, 2024.
25. ClinicalTrials.gov. A study to optimize subretinal surgical delivery and to evaluate safety and activity of Opregen in participants with geographic atrophy secondary to age-related macular degeneration. https://clinicaltrials.gov/study/NCT05626114. Accessed February 2, 2024.
26. ClinicalTrials.gov. Long term follow up of sub-retinal transplantation of hESC derived RPE cells in patients With AMD. https://clinicaltrials.gov/study/NCT02463344. Accessed February 2, 2024.
27. ClinicalTrials.gov. Study of subretinal implantation of human embryonic stem cell-derived RPE cells in advanced dry AMD. https://clinicaltrials.gov/study/NCT02590692. Accessed February 2, 2024.
28. Kashani AH, Uang J, Mert M, et al. Surgical method for implantation of a biosynthetic retinal pigment epithelium monolayer for geographic atrophy: Experience from a Phase 1/2a study. Ophthalmol Retina 2020;4:3:264-273.
29. Humayun MS, Clegg DO, Dayan MS, et al. Long-term follow-up of a Phase 1/2a clinical trial of a stem cell-derived bioengineered retinal pigment epithelium implant for geographic atrophy. Ophthalmology Dec 30, 2023. [Epub ahead of print].
30. Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells 2014;6:2:195-202.
31. ClinicalTrials.gov. A study to evaluate the safety of CNTO 2476 in patients with advanced retinitis pigmentosa. https://clinicaltrials.gov/study/NCT00458575. Accessed February 2, 2024.
32. Spencer R, Fisher S, Lewis GP, Malone T. Epiretinal membrane in a subject after transvitreal delivery of palucorcel (CNTO 2476). Clin Ophthalmol 2017;11:1797-1803.
33. Ho AC, Chang TS, Samuel M, et al. Experience with a subretinal cell-based therapy in patients with geographic atrophy secondary to age-related macular degeneration. American Journal of Ophthalmology 2017;179:67-80.
34. ClinicalTrials.gov. A study to evaluate the safety and clinical response of subretinal administration of CNTO 2476 in participants with geographic atrophy (PRELUDE).
https://clinicaltrials.gov/study/NCT02659098. Accessed February 2, 2024.
35. Heier JS, Ho AC, Samuel MA, et al. Safety and efficacy of subretinally administered Palucorcel for geographic atrophy of age-related macular degeneration: Phase 2b Study. Ophthalmol Retina 2020;4:4:384-393.
36. Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med 2017;376:11:1038-1046.
37. Maeda T, Takahashi M. iPSC-RPE in retinal degeneration: Recent advancements and future perspectives. Cold Spring Harb Perspect Med 2023;13:8:a041308.
38. Hirami Y, Mandai M, Sugita S, et al. Safety and stable survival of stem-cell-derived retinal organoid for 2 years in patients with retinitis pigmentosa. Cell Stem Cell 2023;30:12:1585-1596.e6.
39. da Cruz L, Fynes K, Georgiadis O, et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 2018;36:328–337.
40. ClinicalTrials.gov. Treatment of central retinal vein occlusion using stem cells study (TRUST). https://clinicaltrials.gov/study/NCT03981549. Accessed February 2, 2024.
41. Susanna S Park; Intravitreal autologous bone marrow CD34+ cell therapy for retinal vein occlusion: Phase I/II clinical trial. Invest. Ophthalmol Vis Sci 2018;59:9:3905.
42. ClinicalTrials.gov. Safety of repeat intravitreal injection of human retinal progenitor cells (jCell) in adult subjects with retinitis pigmentosa. https://clinicaltrials.gov/study/NCT04604899?cond=Retinitis%20Pigmentosa&aggFilters=status:com&term=cell&rank=3. Accessed February 2, 2024.
43. ClinicalTrials.gov. CNS10-NPC for the treatment of RP. https://clinicaltrials.gov/study/NCT04284293?cond=Retinitis%20Pigmentosa&aggFilters=status:rec&page=2&rank=12. Accessed February 2, 2024.
44. First U.S. patient receives autologous stem cell therapy to treat dry AMD. https://www.nei.nih.gov/about/news-and-events/news/first-us-patient-receives-autologous-stem-cell-therapy-treat-dry-amd. Accessed February 1, 2024.



