Uveal melanoma is the most common primary malignancy of the eye. About 90 percent of these tumors arise from the ciliary body or choroid (posterior uveal melanoma), whereas about 10 percent arise from the iris. In recent years, there have been significant improvements in diagnostic capabilities and therapeutic options. This update will highlight recent advances and controversies in uveal melanoma management. The choice of treatment for individual patients will depend on many factors, including tumor size and location, and patient age, health, and life expectancy.
Iris Melanomas
For iris melanomas, the most significant recent advance has been high frequency (20 MHz to 50 MHz) anterior segment ultrasonography (See Figure 1). This modality allows us to accurately measure tumor thickness, detect retroiridal tumor extension, and evaluate iris cysts, which can be associated with an occult melanoma.
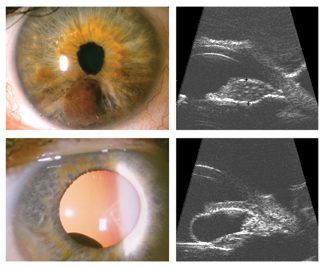 |
| Figure 1. High-frequency anterior segment ultrasound (HFUS) has improved our ability to evaluate iris tumors. Top, HFUS showed that an iris melanoma was limited to the iris and did not extend into the ciliary body. Bottom, HFUS confirmed that a retroiridal mass was an isolated, cystic lesion with internal acoustic hollowness, consistent with an iris pigment epithelial cyst. |
The most common treatments for iris melanoma are iridocyclectomy (for small, localized tumors) and enucleation (for larger tumors and those with dispersion into the angle). Plaque radiotherapy was recently shown to be a possible alternative, however, especially for unresectable tumors that would otherwise require enucleation. In studies using Iodine-125 or Palladium-103, plaque therapy not only provided good tumor control, but it caused fewer anterior segment complications than expected. Additional studies are needed to determine whether plaque therapy may also be useful in resectable tumors.
Management
Since the management of posterior uveal melanomas depends to a great extent on tumor size, we consider small, medium and large tumors separately. For small choroidal tumors—defined by the Collaborative Ocular Melanoma Study (COMS) as 1 to 3 mm in thickness and 5.0 to 16 mm in largest diameter—the most controversial question is when to treat.
The basic problem is that we cannot determine precisely by clinical examination when a uveal melanocytic lesion undergoes malignant transformation. We typically assume that growth is a reasonable indicator of malignancy, and most small tumors are monitored for growth prior to treatment. Clinical features shown to predict growth include subretinal fluid, orange pigmentation, visual changes, thickness more than 2 mm, lack of drusen, and lack of surrounding chronic RPE changes. Subretinal fluid, which is one of the strongest predictors of growth, can be difficult to distinguish clinically from retinal atrophic changes over a dormant tumor. We have found optical coherence tomography to be very helpful in identifying and quantifying active subretinal fluid (See Figure 2).
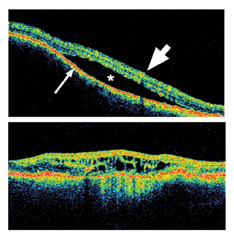 |
| Figure 2. Optical coherence tomography can aid in diagnosing subretinal fluid associated with an active choroidal melanoma. Top, when separation of the retina overlying a choroidal tumor (asterisk) is due to subretinal fluid from an active melanoma, the retinal layers (large arrow) and RPE (small arrow) usually have a normal appearance. Bottom, when retinal separation is due to chronic retinal changes overlying a dormant choroidal nevus, the retinal layers are often disorganized and atrophic, and the RPE is thickened and irregular (bottom). |
Since up to 31 percent of untreated small tumors will grow within five years, does waiting for growth prior to treatment increase the risk of metastasis? In the COMS small tumor observational trial, only 3 percent of untreated patients died of known metastatic disease, and it cannot be determined from the study whether prompt treatment would have altered their outcome. In several retrospective studies, observing small choroidal melanocytic tumors for growth prior to treatment did not significantly increase the metastatic rate.
In the absence of a randomized, prospective trial, the weight of evidence would probably still suggest that close monitoring of small tumors poses minimal risk for metastasis as long as treatment is instituted promptly once growth occurs.
For medium tumors, the main controversy is the choice of treatment. The COMS randomized medium tumors—defined as 2.5 to 10 mm in thickness and no more than 16 mm in largest diameter—to Iodine-125 plaque radiotherapy versus enucleation. This study recently reported no difference in survival between treatment groups with at least five years of follow-up in 81 percent of patients. This result further establishes plaque radiotherapy as an acceptable alternative to enucleation in appropriate tumors.
Charged particle radiotherapy has been associated with low failure rates of around 2-5 percent, whereas plaque therapy in the COMS had a failure rate of more than 10 percent. In our center and others that routinely use intraoperative ultrasonography to localize plaques, the local failure rates have been as low as those reported for charged particles, suggesting that many plaque failures may be due to poor plaque localization. Thus, we recommend the routine use of ultrasound localization in most cases (See Figure 3).
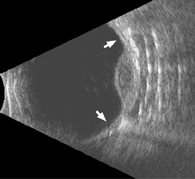 |
| Figure 3. Intraoperative ultrasonography is very useful for localizing radioactive plaques and probably reduces the local failure rate of plaque therapy. This sonogram shows that the plaque is well centered around a dome-shaped choroidal melanoma. The arrows indicate the plaque edges. |
Lightly pigmented and thicker tumors are particularly refractory to TTT. As a result, we now limit primary TTT to heavily pigmented tumors less than 2.5 mm thick and more than 2 mm from the fovea. Despite these shortcomings, TTT has proven to be beneficial as an adjunct following plaque radiotherapy. We currently use TTT after plaque radiotherapy for juxtapapillary tumors (which are at higher risk for local failure) and when tumors fail to respond promptly to plaque radiotherapy.
The COM randomized large tumors—defined as more than 10 mm in thickness or at least 16 mm in largest diameter—to enucleation, with or without preoperative external beam radiotherapy. No difference in survival was noted between treatment groups. As a result, enucleation without adjuvant radiotherapy is currently the most common treatment for large tumors in the United States. However, other treatment options are available for selected circumstances (e.g., one-eyed patients).
Plaque radiotherapy is effective in many cases, albeit with a high rate of complications such as neovascular glaucoma (21 percent) and subsequent enucleation (34 percent). Transscleral cyclochoroidectomy has showed similar local control and better visual outcomes compared to Iodine-125 plaque radiotherapy.
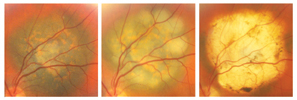 |
| Figure 4. Transpupillary thermotherapy may be effective as a primary therapy for selected small choroidal melanomas. These pictures show a small melanoma prior to treatment (left), immediately after treatment (middle), and one year later (right), with replacement of the tumor with an atrophic chorioretinal scar. |
Several other treatments have been proposed for posterior uveal melanomas, including cryotherapy, endoresection, stereotactic radiotherapy, and various combinations of the above. As a note of caution, most of these alternative treatment modalities have not been evaluated in well-controlled, long-term, prospective studies, and we remain unsure how they compare to plaque therapy and enucleation in terms of local tumor control and survival.
Metastatic disease remains a major challenge for which we have made little progress. The mean survival for metastatic disease is only about seven months. The main problem is that metastatic melanoma cells are extraordinarily resistant to conventional chemotherapy. This resistance cannot be completely explained by multidrug resistance mechanisms such as overexpression of P-glycoprotein. One potential explanation is that uveal melanoma cells are intrinsically resistant to apoptosis.
Our research group and others have shown that this resistance may be due, at least in part, to overexpression of anti-apoptotic molecules such as MDM2 and Bcl2. Based on these findings, we are developing therapeutic agents that target these molecules and induce apoptosis selectively in melanoma cells (See Figure 5).
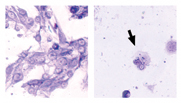 |
| Figure 5. Custom-designed therapeutic molecules may some day improve ocular and systemic outcomes for uveal melanoma patients. These pictures demonstrate the effect of a therapeutic peptide specifically designed to activate the tumor suppressor p53. Melanoma cells treated with a control peptide (left) remained viable and grew rapidly. In contrast, melanoma cells treated with the p53 peptide (right) rapidly died. The arrow indicates a cell in the process of undergoing apoptosis. |
Our research group found that cDNA microarray analysis, which simultaneously examines more than 40,000 genes, may be useful for identifying tumor characteristics such as radiation sensitivity, invasiveness and metastatic potential. Research has shown that fluorescence in situ hybridization on fine needle biopsy specimens can be used to detect loss of chromosome 3, a potential marker for metastatic disease. Eventually, molecular information may be obtained non-invasively using molecular imaging techniques. This information will then be used to formulate an individualized treatment plan.
In patients at high risk for metastasis, effective prophylaxis may be available using immunotherapeutic techniques for activating the immune system against melanoma cells. Metastatic disease may be detected at an earlier stage as a result of serologic surveillance tests based on genomic or proteomic technology.
Effective treatment for metastatic disease may be available in the form of novel molecular agents that directly target melanoma cells and sensitize them to chemotherapeutic agents. Ultimately, the ocular outcomes and life expectancy of uveal melanoma patients may be significantly improved.
Dr. Harbour is an associate professor of Ophthalmology, Molecular Oncology & Cell Biology, and is the director of the Ocular Oncology Service at Washington University School of Medicine. Contact him at Box 8096, 660 South Euclid Ave., St. Louis, Mo. 62110; (314) 747-1738; (fax) (314) 747-5073; e-mail: harbour@vision.wustl.edu.
1. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre- enucleation radiation of large choroidal melanoma II: initial mortality findings. COMS report no. 10. Am J Ophthalmol 1998;125:779-796.
2. Bechrakis NE, Bornfeld N, Zoller I, Foerster MH. Iodine 125 plaque brachytherapy versus transscleral tumor resection in the treatment of large uveal melanomas. Ophthalmology 2002; 109:1855-1861.
3. Char DH, Kroll S, Phillips TL, Quivey JM. Late radiation failures after iodine 125 brachytherapy for uveal melanoma compared with charged-particle (proton or helium ion) therapy. Ophthalmology 2002;109:1850-1854.
4. Diener-West M, Earle JD, Fine SL, Hawkins BS, Moy CS, Reynolds SM, Schachat AP, Straatsma BR. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol 2001;119:969-982.
5. Finger PT. Plaque radiation therapy for malignant melanoma of the iris and ciliary body. Am J Ophthalmol 2001;132:328-335.
6. Harbour JW, Worley L, Ma D, Cohen M. Transducible peptide therapy for uveal melanoma and retinoblastoma. Arch Ophthalmol 2002;120:1341-1346.
7. Harbour JW, Murray TG, Byrne SF, Hughes JR, Gendron EK, Ehlies FJ, Markoe AM. Intraoperative echographic localization of iodine 125 episcleral radioactive plaques for posterior uveal melanoma. Retina 1996;16:129-134.
8. Mueller AJ, Freeman WR, Schaller UC, Kampik A, Folberg R. Complex microcirculation patterns detected by confocal indocyanine green angiography predict time to growth of small choroidal melanocytic tumors: MuSIC Report II. Ophthalmology 2002; 109:2207-2214.
9. Patel KA, Edmondson ND, Talbot F, Parsons MA, Rennie IG, Sisley K. Prediction of prognosis in patients with uveal melanoma using fluorescence in situ hybridisation. Br J Ophthalmol 2001;85:1440-1444.
10. Shields CL, Cater J, Shields JA, Chao A, Krema H, Materin M, Brady LW. Combined plaque radiotherapy and transpupillary thermotherapy for choroidal melanoma: tumor control and treatment complications in 270 consecutive patients. Arch Ophthalmol 2002;120:933-940.