Workup, Diagnosis and Treatment
In the emergency room, an MRI of the brain and orbits revealed focal enhancement along the right posterior globe at the right optic nerve insertion, enhancement of the superior and lateral extraconal area of the right orbit, and curvilinear enhancement of the superior extraconal left orbit. Carotid doppler ultrasound was within normal limits, while orbital doppler U/S showed increased vascularity superiorly and medially in the right orbit. Laboratory workup demonstrated a normal white blood cell count, hemoglobin, platelets, creatinine, sedimentation rate and C-reactive protein.
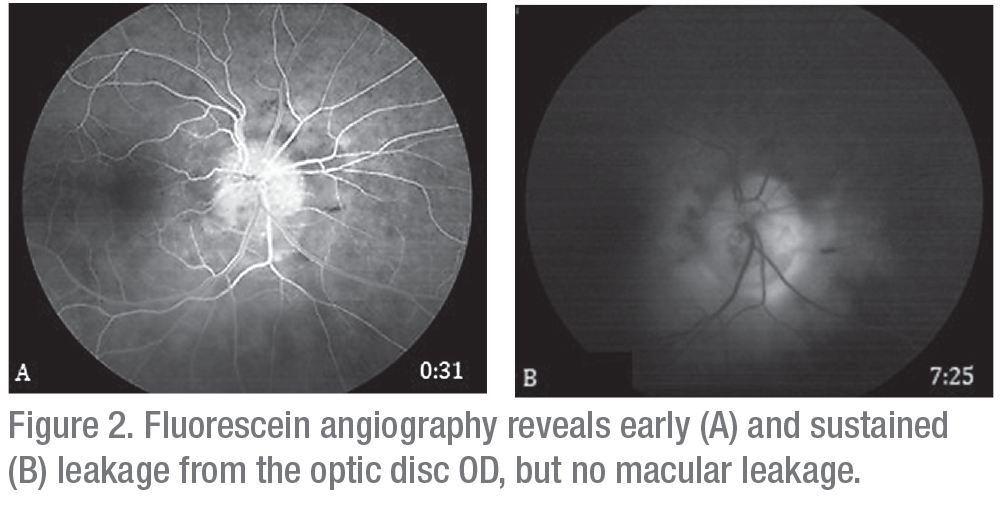 |
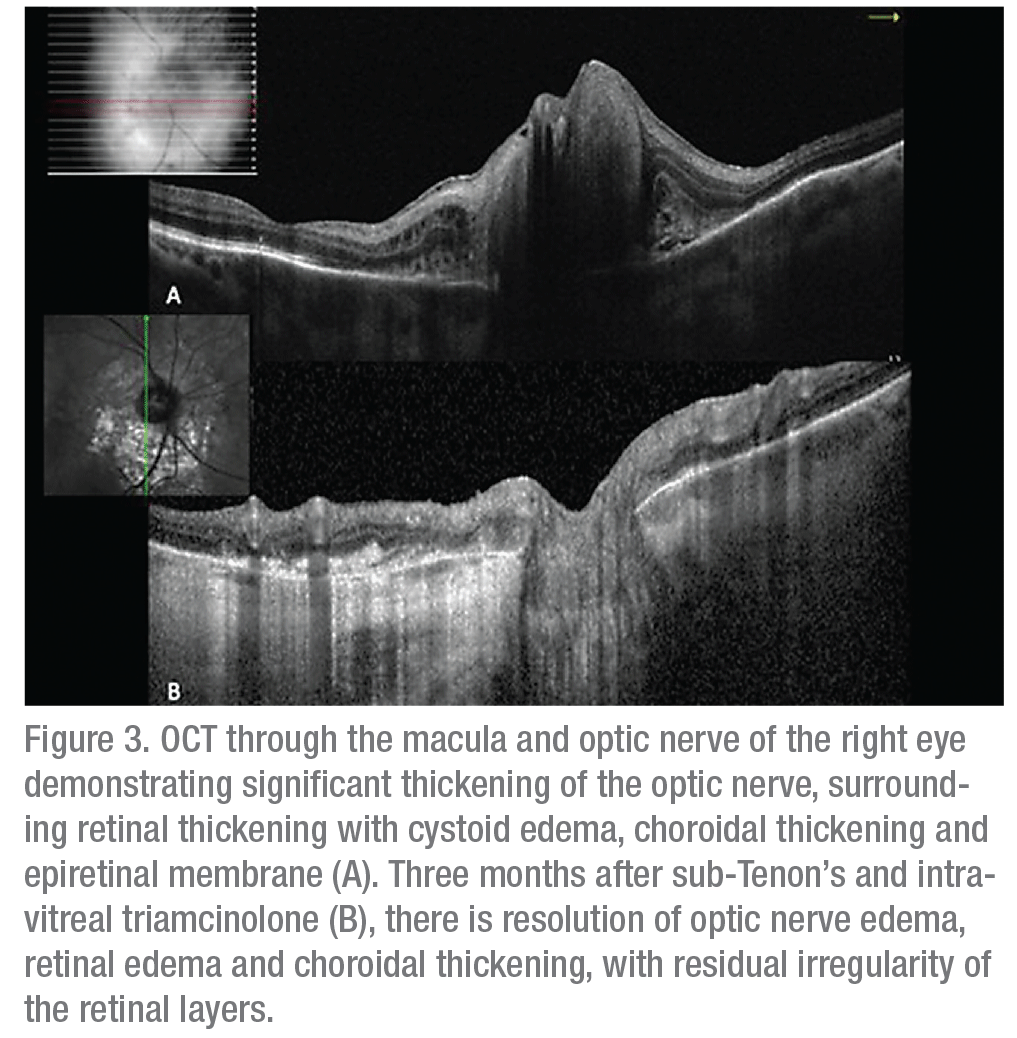
Ancillary imaging was obtained, including fluorescein angiography (Figure 2) and optical coherence tomography (Figure 3). FA revealed leakage from the optic disc of the right eye without macular leakage; a late frame of the left eye was normal. OCT OD demonstrated significant thickening of the optic nerve with adjacent intraretinal edema, peripapillary choroidal thickening, epiretinal membrane and disruption of the ellipsoid zone (Figure 3A). Ultrasound of the right eye showed choroidal thickening surrounding the optic disc with no evidence of extraocular extension.
 |
The differential diagnosis of this patient, with progressive, unilateral vision loss with optic disc edema, surrounding retinal whitening and choroidal infiltration, included infectious, inflammatory and neoplastic etiologies. Possible infectious causes included bacterial (e.g., tuberculosis, syphilis, mycobacteria, Lyme disease), viral (e.g., herpes), fungal (e.g., histoplasmosis), protozoan (e.g., toxoplasmosis) and nematodal (e.g., Toxocara). Inflammatory etiologies included sarcoidosis, giant cell arteritis, Behçets’ and systemic lupus erythematosus. Neoplastic etiologies included lymphomatous or leukemic infiltration of the optic nerve, amelanotic melanoma and metastatic disease.
Angiotensin converting enzyme and a Lyme titer were within normal limits. However, given the appearance of the lesions and the clinical presentation, a preliminary diagnosis of optic nerve granuloma secondary to sarcoidosis was made. Vitreous biopsy was considered but wasn’t pursued given the likely low yield, given the small amount of vitreous cell. The patient was started on oral prednisone 60 mg, which was stopped when she developed hyperglycemia, weight gain and polyuria. To avoid systemic steroids, the patient received a sub-Tenon’s injection of Kenalog. One month later, fundus exam showed improved disc edema, intraretinal edema and retinal hemorrhages (Figure 1A & B and Figure 3B). The patient subsequently received an intravitreal injection of triamcinolone 2 mg. Visual acuity remained stable at 20/40. Fundus exam showed continued resolution of edema (Figure 1C).
Discussion
Sarcoidosis is a systemic inflammatory disease that causes non-caseating granulomas throughout the body.1 The etiology is unknown. Common systemic manifestations include bilateral hilar adenopathy, pulmonary reticular opacities, skin lesions and joint involvement. Other sites of involvement include the liver, spleen, parotid/salivary glands, heart, kidneys and muscles.1 Although pulmonary involvement is the most common manifestation of sarcoidosis, 30 percent of patients present with extrapulmonary disease.2 The incidence ranges from 15.3 to 21.7 per 100,000.1 The lifetime incidence is 1.3 percent in women, 1 percent in men, 2.4 percent in African Americans and 0.8 percent in Caucasians.1
Ocular manifestations occur in 30 to 60 percent of patients and may develop in the absence of other systemic symptoms.3 Uveitis is the most common manifestation of ocular sarcoidosis. The International Workshop for Ocular Sarcoidosis developed a consensus for seven signs suggestive of ocular sarcoidosis.3 They include:
• mutton fat keratic precipitates, small granulomatous keratic precipitates, and iris nodules;
• trabecular meshwork nodules or tent-shaped peripheral anterior synechiae;
• vitreous opacities displaying snowballs or “strings of pearls”;
• multiple chorioretinal peripheral lesions;
• nodular and/or segmental periphlebitis with or without candlewax dripping and/or retinal macroaneurysm;
• optic disc nodule(s),granuloma(s), and/or solitary choroidal nodules; and
• bilateral disease.3
The consensus criteria also address ancillary testing, including: negative tuberculin skin test in a BCG-vaccinated patient or in a patient who has a previously positive tuberculin skin test; elevated angiotensin converting enzyme or serum lysozyme; abnormal liver function test; chest X-ray showing bilateral hilar lymphadenopathy; and positive chest CT in patients with a negative chest X-ray.3
Additionally, IWOS proposed diagnostic criteria. Biopsy-proven disease showing non-caseating granulomas in the setting of a granulomatous uveitis is considered definite ocular sarcoidosis. In the absence of a tissue biopsy, the disease can be considered presumed, probable or possible ocular sarcoidosis depending on which of the above criteria are satisfied.3
Uveitis in sarcoidosis is generally bilateral, with anterior uveitis occurring in 22 to 70 percent of cases.4 Intermediate and posterior uveitis are less common, but posterior disease is still found in up to 28 percent of ocular sarcoidosis cases.1 Ocular sarcoidosis can also involve the lids and orbits. Eyelid involvement can cause painless nodular elevations, while lacrimal gland involvement causes granulomatous inflammation within the gland, leading to disruption of proper tear production and chronic dry eye. Diffuse orbital involvement can cause ptosis, limited extraocular movements and diplopia.4
Optic nerve granulomas are rare but very specific for intraocular sarcoidosis, estimated to occur in 5 percent of all sarcoid patients; one-third of these patients will have other signs of CNS involvement.4,5 Signs and symptoms of optic nerve involvement include rapidly decreasing visual acuity, decreased color vision and diminished contrast sensitivity.4 In a case series of 11 patients with sarcoidosis of the optic nerve, only two of the 11 individuals had previously known sarcoidosis.6 All patients had histologically confirmed non-caseating granulomatous inflammation. ACE levels were tested in three patients and elevated in only one. All patients were treated with oral corticosteroids, and seven of the 11 patients showed improvement in acuity.6
Corticosteroids are the main treatment for ocular sarcoidosis.1 Treatment administration routes include topical, subconjunctival, sub-Tenon’s, peribulbar and intravitreal. Sub-conjunctival dexamethasone can have better penetration than oral steroids.1 Typically, systemic corticosteroid use is reserved for bilateral involvement or multi-organ involvement, with initial dosing starting at 0.5-1.0 mg/kg/day.1 Systemic, steroid-sparing agents, including methotrexate and biologics, can also be used.
In conclusion, sarcoidosis can demonstrate a wide array of intraocular manifestations. Optic nerve granulomas are a rare manifestation of ocular sarcoidosis. It’s crucial to eliminate infectious etiologies prior to initiating corticosteroid therapy. Proper ancillary testing and clinical evaluation must be applied in order to make the proper diagnosis. REVIEW
1. Jamilloux Y, Kodjikian L, Broussolle C, et al. Sarcoidosis and uveitis. Autoimmunity Reviews 2014;13:840-849.
2. Ungprasert P, Carmona EM, Utz JP, et al. Epidemiology of sarcoidosis 1946-2013: A population-based study. Mayo Clin Proc 2016;91:183-188.
3. Herbort CP, Rao NA, Mochizuki M. International criteria for the diagnosis of ocular sarcoidosis: Results of the first international workshop on ocular sarcoidosis. Ocul Immunol Inflamm 2009;17:160-169.
4. Bradley D, Baughman RP, Raymond L. Ocular manifestations of sarcoidosis. Semin Resp Crit Care Med 2002;23:543-548.
5. Kelly JS, Green WR. Sarcoidosis involving the optic nerve head. Arch Ophthalmol 1973;89:486-488.
6. Beardsley TL, Brown SVL, Sydnor CF, et al. Eleven cases of sarcoidosis of the optic nerve. Am J Ophthalmol 1984;7:62-77.



