David Wirta, MD, remembers prescribing glaucoma medications consisting of 4% and 8% concentrations of pilocarpine when he was a resident at UCLA. “Back then,” he recalls, “miosis was a side effect of pilocarpine that adversely affected patients when they walked into dark rooms.” Today, he serves as a principal investigator of four topical medications that will soon temper and use these same side effects to help presbyopic patients see better. One of the investigational agents, AGN-190584 from Allergan, should reach the market by the end of this year, and the other, CSF-1 from Israeli-based Orasis Pharmaceuticals, is expected to soon follow. “We’re entering a whole new world,” says Dr. Wirta, owner of the Eye Research Foundation in Newport Beach, California. “Reformulations of pilocarpine (a cholinergic muscarinic receptor agonist) and other miotics will change how we manage presbyopic patients from here on out.”
In this report, ophthalmologists and 12 manufacturers update you on what lies ahead. Half of the companies are developing topical reformulations of generics used in glaucoma treatment and cataract surgery for decades. The drive to meet the needs of 120 million-plus patients in this unique way has attracted more than $100 million in investor support and positioned entrepreneurial executives to blast the internet and airwaves with consumer advertising, supplanting surgical approaches of yesteryear.
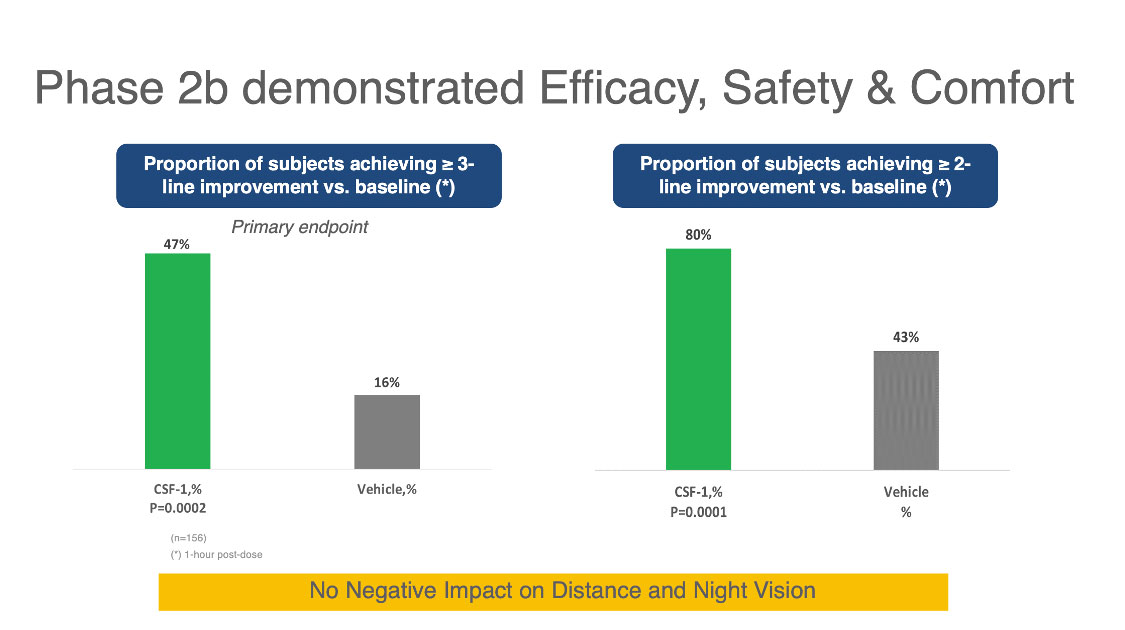 |
| Figure 1. Above are results from a Phase IIb study of the Orasis CFS-1 drop for treating presbyopia. The columns on the left show that 47 percent of subjects using the drop achieved a three-line or better improvement in near vision compared to 16 percent using the vehicle. The columns on the right show that 80 percent of subjects taking the CFS-1 drop achieved a two-line or better improvement in near vision compared to 43 percent using the vehicle. Click image to enlarge. |
New Day Coming
“Pharmacologic treatment of presbyopia is about to meet a great unmet need,” says anterior segment surgeon Eric Donnenfeld, MD, of OCLI Vision, at Island Eye Surgicenter in Westbury, New York. Dr. Donnenfeld is a consultant for Allergan and chairperson of a clinical advisory committee for another developer of topical treatment in this space, Visus Therapeutics in Irvine, California. “Unfortunately, many of the surgical therapies that have been used to treat presbyopia really haven’t panned out. Presbyopic LASIK, scleral inlays and corneal inlays have all been fraught with problems, and we’ve learned that many plano presbyopic patients aren’t willing to undergo these procedures. We need better opportunities for patients. What’s nice about presbyopic drops is that they represent a reversible therapy that patients can stop at any time. The drops’ efficacy appears to be really quite good. They are derived from proven medications with long histories.”
By the end of this year, product developers at Allergan, an AbbVie company, expect the U.S. Food and Drug Administration to act on an NDA filed in February for AGN-190584 (pilocarpine 1.25%) ophthalmic solution, an investigational, novel, optimized formulation of pilocarpine designed as a topical, once-daily drop delivered by a proprietary vehicle. The application is based primarily on data from two Phase III clinical studies (GEMINI 1 and GEMINI 2), which have evaluated the efficacy, safety and tolerability of AGN-190584. The most updated data on AGN-190584—the complete Phase III GEMINI 1 study results—were revealed at the 2021 ASCRS meeting in Las Vegas on July 25.
The GEMINI 1 clinical study evaluated 323 participants randomized in a one-to-one ratio of vehicle (placebo) to AGN-190584. AGN-190584 was instilled bilaterally, once-daily, for 30 days in participants with presbyopia. The primary and key secondary endpoints were met. Compared to those receiving the vehicle, a statistically significantly greater proportion of AGN-190584-treated participants gained three lines or more in mesopic, high-contrast and binocular DCNVA at Day 30, including at hour three (22.5 percent, p<0.0001) and at hour six (9.7 percent, p=0.0114).
The results demonstrated AGN-190584 had a rapid onset of 15 minutes and duration of up to six hours in mesopic DCNVA, without loss of distance vision, after administration at Day 30. Additional endpoints showed that 75 percent of participants treated with AGN-190584 achieved a ≥2-line improvement in mesopic DCNVA and that 93 percent of participants achieved ≥20/40 vision in photopic DCNVA. Improvements were also observed in DCIVA up to 10 hours at day 30. No treatment-emergent serious adverse events were observed in AGN-190584-treated participants.
Next in Line?
In terms of possible timing of approval, other entrants in the field include the following:
• CSF-1 (Orasis). Orasis CEO Elad Kedar and industry observers believe that CSF-1, containing a low concentration of pilocarpine (undisclosed at this point) and a proprietary vehicle, will possibly be the second drop to reach the market with FDA approval. The company is currently conducting its Phase III NEAR-1 and NEAR-2 clinical trials, launched in October 2020. Mr. Kedar says the multicenter, double-masked, parallel-group studies, each involving 300 participants, are structured similarly to a Phase IIb study, which showed a gain of at least two lines of near vision in 80 percent of patients and at least three lines of near vision in 47 percent of patients. The Phase IIb study also found no reduction in distance vision or night/low-light vision, he says.
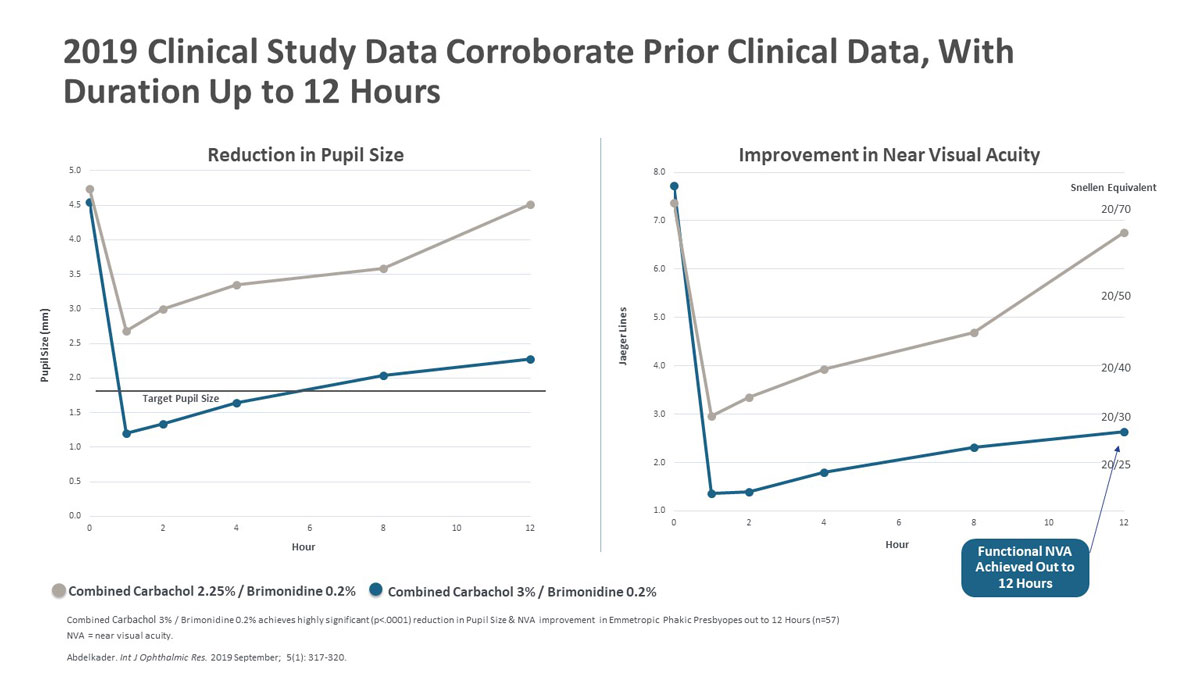 |
| Figure 2. Above are the results from a 2019 clinical study of combined Carbachol 2.25%/Brimodine 0.2% versus combined Carbachol 3%/Brimonidine 0.2%, showing that the stronger concentration maintained a pupil size below 2.5 mm for 12 hours post-treatment while achieving functional near visual acuity that lasted for up to 12 hours. Click image to enlarge. |
Mr. Kedar says the key to success when treating presbyopia by reducing the diameter of pupils is to not burden the patient with excessive miosis, which can negatively affect distance vision, night vision and visual field. By striving for comfort, Mr. Kedar says his team has formulated a drop that minimizes side effects such as headache, brow ache and red eye. “While the specific elements of our vehicle are proprietary, I can say our careful balance allows us to achieve the desired therapeutic effect in a concentration significantly below the glaucoma range for pilocarpine,” he says.
With respect to a potential launch date, Mr. Kedar adds: “We’re currently focused on completing the ongoing Phase III trials, with the data readout anticipated later this year. FDA submission and review will follow shortly thereafter.”
• Brimochol (Visus Therapeutics). Unlike other topical presbyopic treatments, Brimochol is a combination product with two active ingredients—the parasympathomimetic carbachol and the selective alpha-2 adrenoceptor agonist brimonidine tartrate. Rhett Schiffman, MD, MS, MHSA, chief medical officer and head of R&D at Visus, notes that carbachol, mimicking the effect of acetylcholine on the muscarinic and nicotinic receptors, has been used to decrease IOP in glaucoma patients by inducing miosis for more than 50 years. It’s also marketed as an injectable formulation to constrict the pupil during cataract surgery.
Brimonidine blocks the dilation of the pupil under dark conditions and appears to inhibit some of the adverse effects of carbachol or pilocarpine by inhibiting alpha-2 receptors on the ciliary body, Dr. Schiffman adds. “We also have good evidence that brimonidine increases the half-life of carbachol,” says Dr. Schiffman. “Previous published data indicate that this combination product can have very beneficial effects on your visual acuity for eight to 12 hours. A single dose in the morning would last you all day.”
Another feature of Brimochol that may attract patient interest is that brimonidine, the active agent in Lumify, reduces ocular redness, which is associated with the use of pilocarpine and carbachol.
Visus initiated its Phase II study of Brimochol in March 2021 to evaluate the safety, tolerability and efficacy of two formulations, Brimochol and Brimochol Free (for dry eyes) in a three-arm crossover study, according to Dr. Schiffman. “Visus estimates FDA approval in Q4 2024 if everything goes as planned,” he says.
Push of a Button
New York City-based Eyenovia, a clinical stage ophthalmic biopharmaceutical company, is developing a proprietary pilocarpine solution called MicroLine that’s administered in a “microdose,” minimizing adverse effects. Eyenovia’s unique proprietary Optejet spray dispenser, featuring an automated push-button function, activates a 7-ml burst of the cholinergic muscarinic receptor agonist, reducing potential systemic exposure and toxicity, according to the company. MicroLine’s Phase III VISION-1 clinical trial found that fewer than 3 percent of study participants reported headache and brow ache, compared to a 20 to 25 percent incidence observed in some other studies of eye drop formulations of pilocarpine.
“A typical eye drop may be 20 to 50 ml, depending on viscosity,” says Dr. Wirta, a principal clinical investigator of MicroLine. “Because only 7 ml of the formulation is delivered by Eyenovia’s Optejet dispenser, this formulation doesn’t spill onto the patient’s face and beyond the targeted area of the eye. Although miosis by itself can still contribute to adverse effects, this lower dose may be a factor in MicroLine’s reduced adverse effects. The effect of the treatment peaks one hour after administration, providing up to three lines in improved vision. This is an on-demand treatment designed to be used with readers or prescribed lenses, as needed, and it provides improved vision up to three hours or more, depending on individual response.”
 |
| Figure 3. Eyenovia’s Optejet spray dispenser, if approved by the FDA, would enable presybopes to self-administer an automated 7-ml burst of pilocarpine with the push of a button, as needed, without having to tilt their heads back for instillation of a drop. The small dose is also designed to reduce potential systemic exposure and toxicity. |
Eyenovia’s VISION-1 study evaluated the safety and efficacy of the company’s 1% and 2% pilocarpine Micro-Array Print (MAP) formulations versus placebo, all administered with the Optejet dispenser. A higher proportion of subjects met the primary endpoint of three-line or greater improvement in near vision with 2% MicroLine, compared to placebo (Odds Ratio=7.7; statistically significant difference p<0.05). A higher proportion of subjects achieved two-line or greater improvement in near vision with 2% MicroLine as compared to placebo (Odds Ratio=10.8, statistically significant difference p<0.05). Eyenovia plans to initiate a second Phase III registrational trial, VISION-2, later this year.
Unique Approach
Lenz Therapeutics of San Diego, formerly called Presbyopia Therapies, has reorganized and eliminated the previous trade name of its developmental product, whose active ingredient, aceclidine, is a parasympathomimetic agent used in the treatment of open-angle glaucoma as a topical drop.
Steven J. Dell, MD, a Lenz Therapeutics scientific advisory board member and investor in the company, says a 1998 study indicates that aceclidine has the potential to function as a best-in-class treatment of presbyopia by uniquely targeting the iris sphincter.1 The muscarinic acetylcholine receptor agonist demonstrates the ability to stimulate the pupil with minimal effect on the ciliary muscle, compared to the more significant effects on the ciliary muscle created by pilocarpine and carbachol, the other active ingredients in today’s emerging topical presbyopia treatments, according to Dr. Dell, who is also the medical director of Dell Laser Consultants in Austin, Texas.
He notes that aceclidine, pilocarpine and carbachol stimulate the iris sphincter to create a pinhole effect, which is desirable, but not all three spare the ciliary muscle significant and undesirable stimulating effects. Strong stimulation of the ciliary muscle is undesirable in the treatment of presbyopia because it causes lens shift, increased lens thickness and varying induced levels of myopia, he continues. “For example, only 900 nmol/L of aceclidine triggers the sphincter muscles, but 22 times that amount would be required to trigger the ciliary longitudinal muscle,” he says. “This is a high independence ratio versus what is found in the actions of the other miotics.”
In 1977, he adds, researchers used these three miotics to elicit a pinhole effect and measured the corresponding impact on the lens created by the ciliary muscle, echoing the impact on the ciliary muscle found in the 1998 study.2
Dr. Dell says Lenz Therapeutics’ aceclidine product will be a daily eye drop. “We completed a successful Phase II study,” he says. “The product was well-tolerated. The most common side effect was mild discomfort on instillation and there were no serious adverse events. Our current focus is on Phase III clinical development to enable FDA submission and bring the product to market.”
Second Combination Drug
Another emerging topical presbyopic treatment combines phentolamine ophthalmic solution 0.75% and low-dose 0.4% pilocarpine, according to the United States National Library of Medicine’s clinical trial website. The product in development is Nyxol, manufactured by Ocuphire Pharma in Farmington Hills, Michigan.
A Phase II interventional trial that currently involves 150 randomized, parallel-assigned participants, with quadruple masking (participant, care provider, investigator and outcomes assessor) was begun on February 15 and is expected to conclude in September 2021, according to the FDA. Participants qualified for the trial if they had near visual acuity of 20/50 or worse.
Ocuphire says the primary endpoint of the trial is the percentage of patients with at least three lines (15 letters or more) of binocular distance-corrected near visual acuity (DCNVA) improvement on a standard near vision eye chart in photopic lighting conditions. Secondary endpoints at multiple timepoints include improvement of three lines of DCNVA without any loss of distance vision, pupil diameter and improvement in DCNVA of one or two lines when compared to placebo, phentolamine ophthalmic solution 0.75% alone and low-dose pilocarpine alone.
Alternative Mechanisms of Action
Not all topical treatments of presbyopia will be used to stimulate miosis. Novartis’ UNR844 is a prodrug that penetrates the cornea and uses lipoic acid choline ester to reduce disulfide bonds in the lens which, over time, restrict the lens from changing shape via ciliary muscle contraction and relaxation. The age-related disulfide bonds also contribute to the development of nuclear sclerotic cataracts.
| What Happened to Surgery for Presbyopia? Although most early innovators of invasive treatments for presbyopia are in retreat, at least one U.S. company refuses to be counted out. The VisAbility Micro Insert System (Refocus Group, Aliso Viejo, California) consists of four 5.8-mm-long micro-thin polymethylmethacrylate (PMMA) scleral implants, each smaller than a grain of rice. The implants are placed just below the surface of the sclera about 4 mm from the limbus in the four quadrants of both eyes using a specially modified sclerotome system to ensure uniformity. Each implant has two parts; the second, smaller part locks the first part in place inside the scleral tunnel. The VisAbility Micro Insert System is based on a theory that age-related enlargement of the lens decreases space between the lens and ciliary muscle, contributing significantly to presbyopia. The VisAbility system is intended to adjust the tension of the posterior zonules, altering the spacing relationship between these structures. In an IDE clinical study (VIS-2014), 360 presbyopic subjects 45 to 60 years of age had the procedure in the primary eye, followed by surgery in the fellow eye of 348 of the subjects at least 14 days later, according to Mike Judy, CEO of Refocus-Group. The primary endpoint of 20/40 or better DCNVA at 40 cm and at least 10 letters of improvement in the primary implanted eye was achieved in 79.1 percent of primary eyes at 12 months, 84 percent of primary eyes at 24 months. A follow-up study from the completed IDE found 90 percent (52/58) of all eyes maintained 20/40 or better DCNVA. In addition, 67 percent (39/58) achieved 20/32, and 52 percent (30/58) achieved 20/25 or better at the last visit 48 or 60 months postop. Distance vision remained stable in all enrolled eyes. No serious ocular adverse events were reported.1 “VisAbility treatment (under FDA study review) has the potential to provide a permanent solution for treating presbyopia without impacting the lens and cornea, since it’s implanted in the sclera,” says Mr. Judy. Another form of surgery for presbyopia is the Pearl Procedure, developed by Soosan Jacob, MS, FRCS, DNB, director and chief at Dr. Amar Agarwal’s Refractive and Cornea Foundation in Chennai, India. (You can watch a video of the procedure at youtu.be/8H4Ns1b8L3M.) The procedure uses a biological tissue implant (rather than a synthetic implant) to create a hyperprolate central cornea that improves near and distance vision. Dr. Jacobs reports that she plans to continue to increase the use and refinement of this surgery. Meanwhile, two presbyopic surgical approaches once promoted as promising are idle in the United States for now. “CorneaGen has stopped marketing and promoting the KAMRA Inlay,” notes Tami Kelly, Brazer Communications for CorneaGen. The same status applies to Supracor, a procedure developed by Bausch + Lomb that involves the use of laser ablation to create a multifocal cornea. “We aren’t planning to introduce Supracor in the United States at this time,” says Kristy Marks of Bausch + Lomb. 1. 2021 ASCRS Electronic Abstract Submission by Frank Bucci, MD |
The company’s prospective, randomized, double-masked, placebo-controlled Phase I/II study enlisted 75 patients, ages 45 to 55 years, with presbyopia. At baseline, participants’ distance-corrected near vision was below 20/40 in both eyes. Fifty patients received UNR844; 25 received placebo. Patients received drops for 91 days and were then monitored for seven months. According to a company spokesperson who asked to remain anonymous, findings included:
• Bilaterally, 74 percent of patients receiving UNR844 improved to 20/40 or better, versus 33 percent of those receiving placebo. Although 82 percent of patients receiving UNR844 finished the study at 20/40 or better (compared to 48 percent receiving placebo), some of them started with bilateral vision of 20/40 or better. “This is because the inclusion criteria required that monocular vision be worse than 20/40 in each eye,” the spokesperson says. “However bilateral vision can be better than monocular. The 74 percent versus 33 percent numbers here excluded them (subjects with bilateral vision of 20/40 or better) from the analysis.”
• Fifty-three percent of those receiving UNR844 experienced an improvement of at least 0.2 logMAR; only 22 percent receiving placebo achieved this.
The drug caused no change in visual acuity, manifest spherical equivalent or pupil diameter.
• Seven months after study completion, 39 percent of treated subjects maintained their improvement in bilateral vision (defined as an improvement of at least 0.2 logMAR), compared to only 6 percent of the placebo group. A Phase IIb study of UNR844 in patients with presbyopia was initiated in June; the first interpretable results are expected in 2022.
Another unique mechanism of action for presbyopia-correcting therapy is employed by Yolia True Vision, which involves self-administration of proprietary enzyme eye drops that increase corneal malleability, followed by the wearing of individually customized contact lenses that reshape the cornea’s sphericity to produce multifocal vision.
The treatment, approved by the Mexican FDA, Cofepris, is part of Yolia Health’s platform of similar treatments. CEO and co-founder Alberto Osio reports that his company is currently treating emmetropes with presbyopia and post-refractive patients with presbyopia. “We will soon begin testing our myopia progression treatment,” he adds. “We’ve also been successful at creating multifocal corneas and therefore will use the same approach for controlling the progression of myopia. In addition, Yolia has recently entered into a commercial partnership in Asia.”
Benefiting from the U.S. FDA’s willingness to accept data established in Mexico, Mr. Osio says he is hoping for expedited evaluation and approval of his company’s treatments by the FDA in 2023.
Dr. Wirta is a principal investigator of AGN-190584 (Allergan), MicroLine (Eyenovia), Brimochol (Visus Therapeutics) and CSF-1 (Orasis). Dr. Donnenfeld is a consultant for Allergan and chair of the clinical advisory committee for Brimochol. Dr. Schiffman is chief medical officer and head of R&D at Visus Therapeutics. Dr. Dell is a member of the advisory board of and investor in Lenz Therapeutics.
1. Ishikawa H, DeSantis L, Patil PN. Selectivity of muscarinic agonists including (+/-)-aceclidine and antimuscarinics on the human intraocular muscles. J Ocul Pharmacol Ther 1998;14:4:363-73.
2. Francios J, Goes F. Ultrasonographic study of the effect of different miotics on the eye components. Ophthalmologica 1977;175:6:328-38.