Cataract surgeons must operate on eyes with many different characteristics, and sometimes those characteristics make the surgery more challenging. A short eye with narrow angles or angle closure is a case in point.
Our understanding of the nature and consequences of a narrow angle has increased dramatically in the past 20 years. Narrow angles have been the focus of a number of clinical studies, including the Zhongshan Angle Closure Prevention (ZAP) trial that compared the value of doing a laser peripheral iridotomy to not doing an LPI in patients who are primary angle closure suspects; and the EAGLE study, which compared the impact of clear lens extraction in patients with primary angle closure with intraocular pressure 30 mmHg or greater, or primary angle-closure glaucoma, to the impact of performing a laser peripheral iridotomy.
Short eyes with narrow angles have traditionally been challenging in terms of choosing the best intraocular lens power, although today’s more sophisticated formulas and biometers have made this less of an issue. But performing cataract surgery in the presence of a narrow angle can still be tricky. The good news is, you can usually open the narrow angle significantly by removing the cataract. The bad news is, it can be technically challenging to operate in an eye with narrow angles, because the space in which you have to operate is compact.
Here, I’d like to offer some insights regarding the nature of angle closure; discuss the relative merits of performing cataract surgery vs. performing an LPI; share some strategies for improving safety and outcomes when performing cataract surgery on short, narrow-angle eyes; and share some thoughts on preventing and managing malignant glaucoma, which can occur postoperatively in some of these eyes.
Classification of Angle Closure
Whenever we discuss narrow angles it’s important to be clear about our terminology. With that in mind, I’d like to review the current recognized nomenclature relating to angle closure.
The “entry level” for angle closure is “primary angle closure suspect.” This describes an eye in which you see contact between the iris and the trabecular meshwork for at least 180 degrees or more of the angle, but the IOP is normal and you don’t see any signs of peripheral anterior synechiae or glaucoma. (The extent of contact between the iris and trabecular meshwork can be determined by gonioscopy, anterior segment OCT or ultrasound biomicroscopy of the anterior segment.)
The second level is “primary angle closure.” This term is used to describe an eye with 180 degrees or more of iris-trabecular touch, where either the IOP is abnormally high or you see peripheral anterior synechiae (or both). However, the patient doesn’t have optic nerve damage.
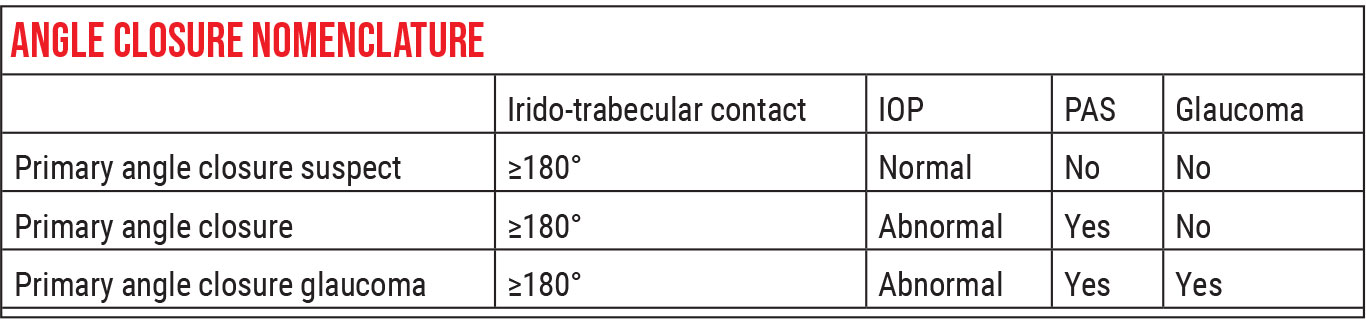 |
The third category, which is straightforward and easy to understand, is primary angle closure glaucoma. This is a description applied to a patient with these abnormalities who does have glaucomatous damage.
LPI vs. Cataract Surgery
Previous studies have clearly shown that performing cataract surgery in any eye can lead to a modest pressure lowering of 2 to 4 mmHg for at least a couple of years. For example, one iStent study, involving 240 eyes with mild-to-moderate glaucoma and an IOP ≥ 24 mmHg, found that while eyes receiving an iStent did better than those not receiving one (72 percent of iStent eyes achieved an unmedicated IOP ≤ 21 mmHg at one year postop, vs. 50 percent of control eyes), half of the control eyes achieved that outcome as a result of the cataract surgery alone.1 Articles reviewing multiple studies that have addressed this question report the same finding,2 as did a review of data from the Ocular Hypertension Treatment Study.3 In eyes with ocular hypertension, cataract removal resulted in a significant lowering of IOP (19.8 ± 3.2 mmHg vs. 23.9 ± 3.2 mmHg; p<0.001).
Interestingly, studies have demonstrated that removing a cataract from eyes with narrow angles can result in an even greater benefit.4,5 IOP reduction following cataract surgery in eyes with primary angle closure glaucoma has been reported in the range of 2 to 12 mmHg.6 Many people believe the explanation for this is a change in the angle anatomy. You can clearly see the deepening of the anterior chamber and widening of the angle in these eyes once the cataract is removed. In fact, this change can be quantified using OCT. Studies have found that cataract surgery increases anterior chamber depth, the width of angle opening and the trabecular iris area.7,8
It’s well known that one way to address some of the potential problems associated with a narrow angle is to perform a laser peripheral iridotomy. The supposition is that because the iris and the lens are in very close contact, pupillary block can occur, trapping fluid behind the iris. (That’s how people go into full-blown angle closure.) When you create the LPI, you’re allowing aqueous humor to equilibrate on both sides of the iris. That allows the iris to flop back a little bit, pushing it away from the wall of the eye. You can often observe this happening after an LPI, although in some cases the LPI may not appear to have much effect. (At least having that hole in the iris will keep the eye from going into an angle closure attack.)
 |
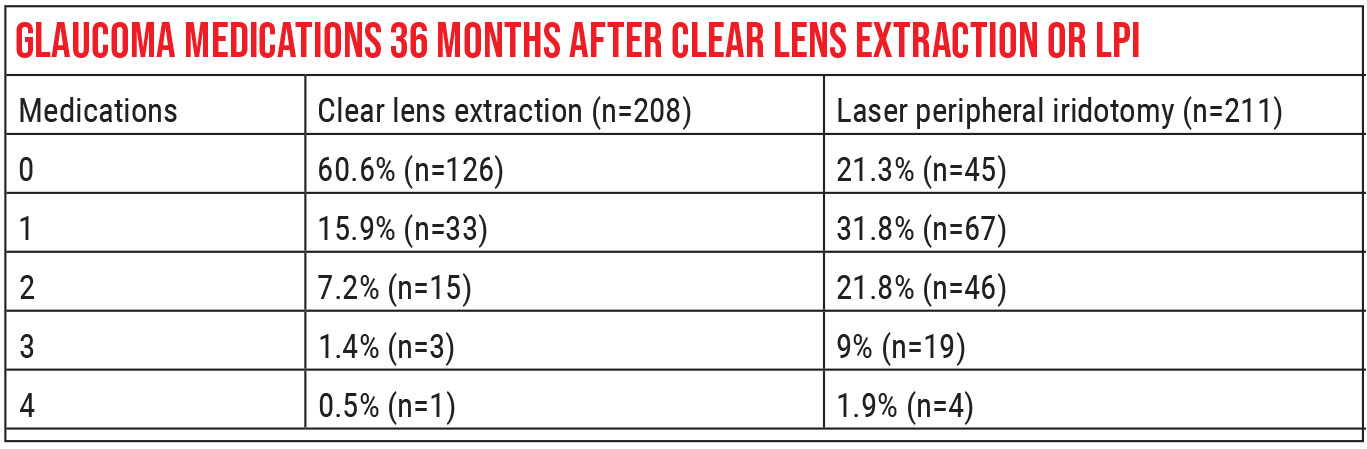
The landmark paper comparing the effectiveness of an LPI to that of cataract surgery is the EAGLE study, which came out in 2016.9 One of the interesting aspects of this study is that they were able to enroll as many subjects as they did—419—because they had very strict criteria for who could be in the study. First, patients had to have primary angle closure with an IOP of 30 mmHg or higher, or primary angle closure glaucoma. (30 mmHg or higher is a pretty abnormal pressure.) Second, participants in the study couldn’t have a cataract.
The participants were randomized to receive either an LPI or clear lens extraction. A significant difference in mean IOP existed at three years postop: The CLE groups averaged a pressure of 16.6 mmHg, which was 1.18 mmHg lower than the average for the LPI group (p=0.005). Quality of life scores were also higher for the CLE group. In addition, patients in the CLE group required fewer glaucoma medications and less additional glaucoma surgery. The study authors concluded that clear lens extraction was more clinically effective and cost-effective than an LPI.
One caveat when interpreting the EAGLE study is to note that patients included in this randomized clinical trial were suffering from sequelae of their narrow angles. They either had significantly high IOP or definitive glaucomatous damage. Patients commonly seen by ophthalmologists have narrow angles with normal IOP, no signs of PAS and no glaucomatous damage (classification: primary angle closure suspect); they’re not in the same category as the patients evaluated in the EAGLE study. Therefore, it wouldn’t necessarily be appropriate to offer clear lens extraction in these cases. Also, a high proportion of EAGLE study patients were Asian; they may have less predominant pupillary block as the mechanism of their angle-closure, compared to non-Asian patients.
Preoperative Considerations
Here are some preoperative strategies that will help ensure a good outcome when performing cataract surgery in these eyes:
• Choose your lens power formula wisely. The prediction of the effective lens position in eyes with short axial length can be harder to estimate than in an eye with average axial length. Third- and fourth-generation intraocular lens formulae such as the Haigis, Holladay II and Hoffer Q are popular choices for short eyes.10 The Barrett Universal II formula can also be helpful, although for very short eyes, it’s advisable to review predictions from multiple formulae and choose an average for the final IOL power. (For more on use of formulas in these situations, see “IOL Power Formulas: 10 Questions Answered” in the January 2018 issue of Review.)
• Set patient expectations. When the eye is shorter than average, one should counsel the patient: “You have a very short eye, so our prediction of the best lens power to give you the vision you want will be less accurate. You could end up a little more farsighted or nearsighted than we intended.” Without setting patient expectations appropriately, you can end up with a very unhappy patient.
• Ask about a history of trauma and look for signs of lens instability. If an eye has already undergone an acute angle-closure attack, the zonules may be unstable; this could cause the lens to drop during a complex cataract surgery with zonular dialysis.
 |
Interestingly, I’ve always assumed that in this situation the preceding angle closure attack caused the lens to be unstable. But a study published in 2017 offers some evidence that the reverse may be true: Lens instability may have caused an acute angle closure attack.1
The authors of this paper present evidence that suggests that loose zonules may have allowed the lens to move forward, leading to the angle closure. They note that in eyes that had a history of acute angle closure and subsequent cataract surgery in which zonular instability was present, a longer axial length and a less-hyperopic spherical equivalent were also present. Normally, the risk of angle closure is associated with being more hyperopic and having a shorter axial length. The authors surmised that the unexpected biometrics of these eyes with loose zonules provide indirect evidence that zonular abnormality preceded the acute angle closure event.
Intraoperative Pearls
 |
Once you’re in the OR, these protocols will increase the likelihood of a good outcome:
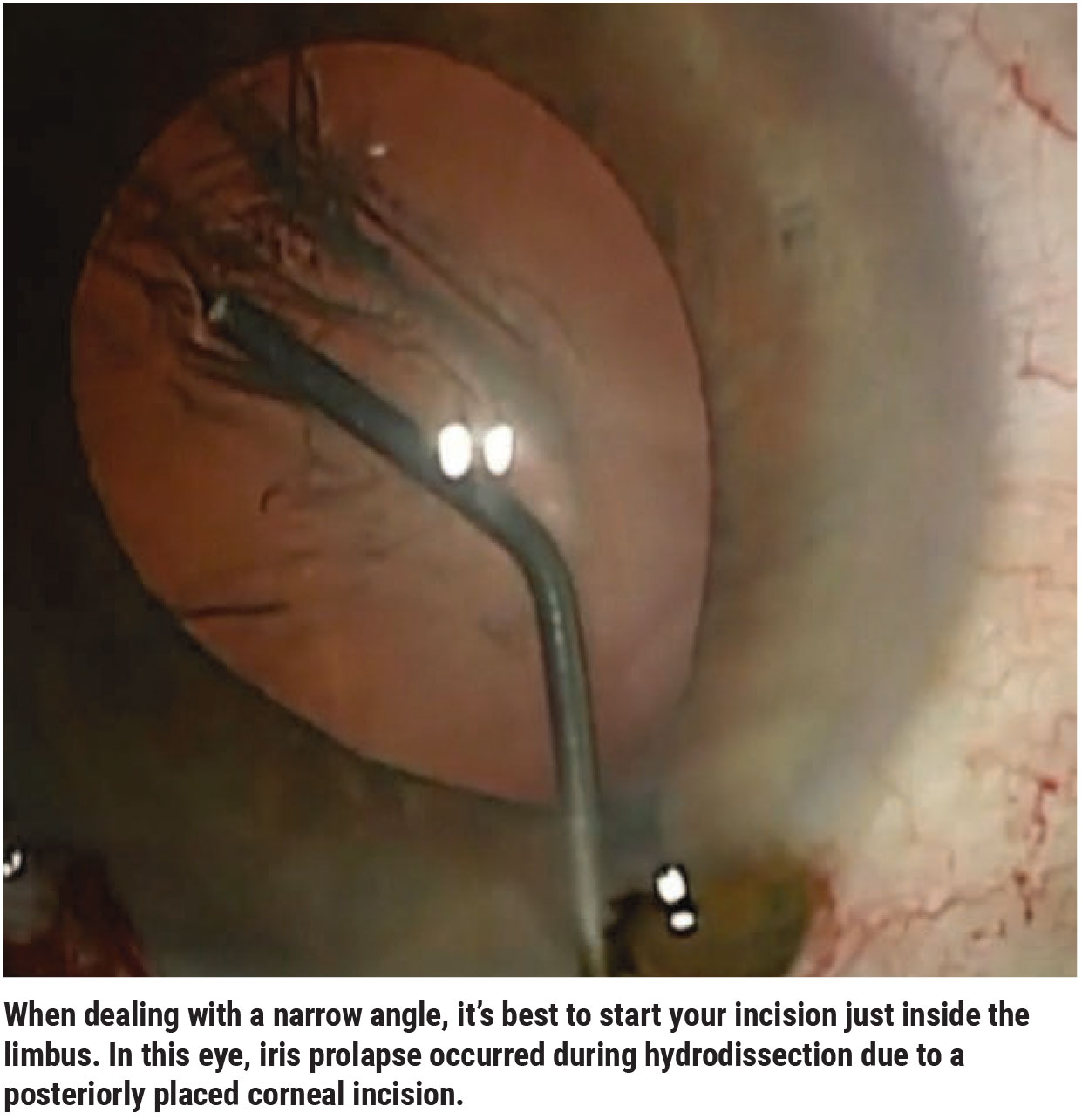
• Start your incision just inside of the limbus. An incision that begins too peripherally can increase the risk of iris prolapse (See image, above) in an eye with a very narrow angle.
• Create a two-step incision. When I enter the eye to make the incision, I point the tip of the blade upwards a little bit to follow the curvature of the cornea; then I change direction and flatten out the blade before entering the anterior chamber. Emphasizing a two-step incision may help prevent iris prolapse.
• If you use a cystotome to make your capsulorhexis, consider putting the needle on your viscoelastic syringe. Many surgeons use a cystotome—a bent hypodermic needle—to tear the capsule. During the capsulorhexis, if pressure is placed on the incision, the viscoelastic can egress, leading to shallowing of the anterior chamber. This can leave you trapped in the eye with a flat chamber and a sharp needle. Having the viscoelastic syringe on the cystotome needle can make it easier to instill more gel in order to reform the anterior chamber.
• Use dispersive viscoelastic during phacoemulsification of the lens. It’s easy to damage corneal endothelium while working in a very tight space. The dispersive viscoelastic can provide a protective coat on the endothelial side of the cornea to reduce the risk of damage.
• Consider intravenous mannitol or acetazolamide. Sometimes one can encounter posterior pressure in short eyes. IV mannitol or acetazolamide can be given to try to decompress that pressure.
• Suture the corneal incision at the end of the case. In an eye with narrow angles, iris prolapse may occur if the corneal incision is disrupted. For example, while instilling eye drops, the patient may accidentally touch the eye with the dropper tip.
• Consider leaving peripheral anterior synechiae alone. Eyes that are narrow can have PAS. Many surgeons do goniosynechialysis to try to open up the trabecular meshwork and get better outflow and lower pressure. This can be done by squeezing viscoelastic at the adhesions to try to break them, by using the cannula of the viscoelastic syringe or a blunt spatula to try to press the adhesions down and strip them away from the walls, or by using micro forceps to try and grasp the PAS.
I used to think that doing this made sense, but I recently read about two randomized clinical trials that found that goniosynechialysis has little effect on IOP lowering.12,13 In addition, the downsides of performing this additional procedure are that it may cause pigment release and bleeding in the eye, while potentially not doing much to improve the IOP outcome.
Preventing Malignant Glaucoma
When performing cataract surgery (or other surgeries) on narrow-angle eyes, postoperative malignant glaucoma is a potential complication, albeit a rare one. A history of angle closure or a short axial length is known to be associated with increased risk of this complication occuring.14
This is more of a concern when the eye is extremely short. For example, a paper by Devesh Varma, MD, and colleagues looked at 20 eyes that developed malignant glaucoma after phacoemulsification surgery.15 The mean axial length in these eyes was 21 ±1.4 mm. (The average eye has an axial length of approximately 23 to 24 mm.) Furthermore, the average refractive error in these eyes was +3 D.
 |
Malignant glaucoma can occur at any time; it can occur intraoperatively, postop day one, postop week one or even later. Usually by the time the patient is a few weeks out the risk diminishes, but it can still occur. Unfortunately, diagnosing malignant glaucoma can be tricky. Signs to look for include:
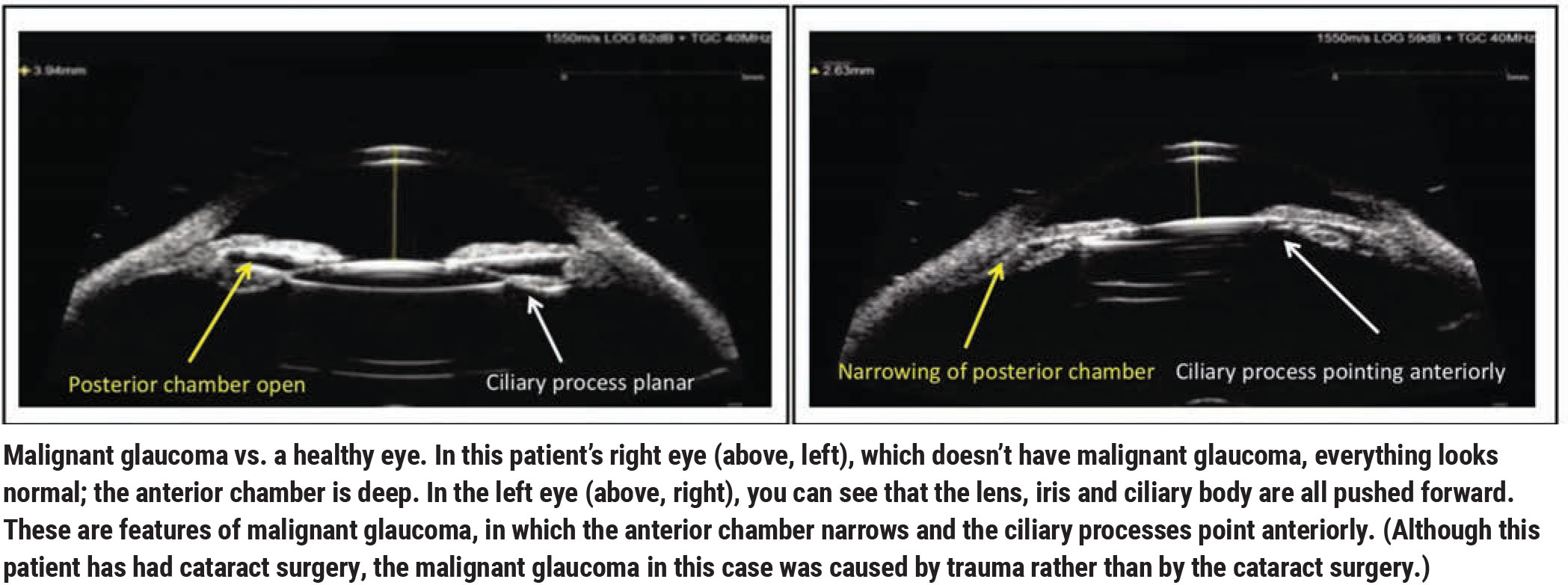
• An asymmetrically shallow anterior chamber. The hallmark of malignant glaucoma is a shallow anterior chamber (See example, above), so failing to see a deeper anterior chamber after removing the cataract should make one at least think about malignant glaucoma as a possible explanation.
• A myopic surprise. If the patient is unexpectedly nearsighted, that means the lens is sitting forward of the predicted lens position. In malignant glaucoma, everything at the lens plane gets pushed forward, including the ciliary body and the lens. (This is true whether the eye is phakic or pseudophakic.) Therefore, unexpected myopia is another warning sign.
• High postoperative pressure. A high postoperative pressure combined with an unexpectedly shallow anterior chamber should raise one’s suspicion for malignant glaucoma. Keep in mind however, that in rare cases, malignant glaucoma can present with a normal IOP, especially if a prior glaucoma procedure such as a trabeculectomy was performed or a glaucoma drainage device is present. The tipoff in this case would be an unexpected shallow anterior chamber.
If I suspect malignant glaucoma, I find that UBM imaging can be helpful in making the diagnosis. One should look for a shallow anterior chamber, narrowing or absence of the posterior chamber, and ciliary processes that may be rotated anteriorly.
Strategies that can help address malignant glaucoma include:
• Consider starting the patient on atropine postoperatively as a precaution. Doing so can decrease the risk of malignant glaucoma. This probably isn’t necessary if the angle is just a little narrow, but if the angle is very narrow preoperatively and the axial length is short, the risk is higher.
• Don’t wait to treat. If malignant glaucoma is present, several maneuvers can be performed to address the problem. First, if the pressure is high, give the patient atropine to induce cycloplegia and start IOP lowering medication. Second, try disrupting the anterior face of the vitreous body with an Nd:YAG laser; this can break an attack. If neither of these options works, a vitrectomy with zonulo-hyaloidectomy is indicated.
Forewarned is Forearmed
As with many non-average eyes, short eyes with narrow angles present challenges to the cataract surgeon. But as our understanding of these eyes continues to improve, the odds of achieving an excellent outcome are better than ever. Hopefully these tips will help your patients end up with excellent vision and minimal postoperative issues.
Dr. Lim is a professor of ophthalmology and vice-chair and medical director of the UC Davis Eye Center. Dr. Lim is an investigator for Santen and has received educational grants from Allergan.
1. Samuelson TW, Katz LJ, Wells JM, et al. US iStent Study Group. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology 2011;118:459–467.
2. Shrivastava A, Singh K. The impact of cataract surgery on glaucoma care. Curr Opin Ophthalmology 2014;25:1:19-25.
3. Mansberger SL, Gordon MO, Jampel H, et al, and the Ocular Hypertension Treatment Study Group. Reduction in intraocular pressure after cataract extraction: The Ocular Hypertension Treatment Study. Ophthalmology 2012;119:9:1826-31.
4. Lai JS, Tham CC, Chan JC. The clinical outcomes of cataract extraction by phacoemulsification in eyes with primary angle-closure glaucoma (PACG) and co-existing cataract: A prospective case series. J Glaucoma 2006;15:47–52.
5. Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): A randomised controlled trial. The Lancet 2016;388:10052:1389-1397.
6. Trikha S, Perera SA, Husain R, Aung T. The role of lens extraction in the management of primary angle-closure glaucoma. Curr Opin Ophthalmol 2015;26:2:128-34.
7. Masis Solano M, Lin SC. Cataract, phacoemulsification and intraocular pressure: Is the anterior segment anatomy the missing piece of the puzzle? Progress in Retinal and Eye Research 2018;64:77-83.
8. Yang HS, Lee J, Choi S. Ocular biometric parameters associated with intraocular pressure reduction after cataract surgery in normal eyes. Am J Ophthalmol 2013;156:1:89-94.e81
9. Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): A randomised controlled trial. The Lancet 2016;388:10052:1389-1397.
10. Xia T, Martinez CE, Tsai LM. Update on Intraocular Lens Formulas and Calculations. J Ophthalmol (Phila) 2020;9:3:186-193.
11. Kwon J, Sung KR. Factors associated with zonular instability during cataract surgery in eyes with acute angle closure attack. Am J Ophthalmology 2017;183:118-124.
12. Moghimi S, Latifi G, ZandVakil N, et al. Phacoemulsification versus combined phacoemulsification and viscogonioplasty in primary angle-closure glaucoma: A randomized clinical trial. J Glaucoma 2015;24:8:575-582.
13. Lee CK, Rho SS, Sung GJ, et al. Effect of goniosynechialysis during phacoemulsification on IOP in patients with medically well-controlled chronic angle-closure glaucoma. J Glaucoma 2015;24:6:405-409.
14. Kaplowitz K, Yung E, Flynn R, Tsai JC. Current concepts in the treatment of vitreous block, also known as aqueous misdirection. Surv Ophthalmol 2015;60:3:229-41.
15. Varma DK, Belovay GW, Tam DY, Ahmed IK. Malignant glaucoma after cataract surgery. J Cat Refract Surg 2014;40:11:1843-9.



