Glaucoma is the leading cause of irreversible vision loss, and the number of Americans with glaucoma is estimated to increase to 3.3 million by the year 2020. Initial therapy usually consists of topical medications or laser treatment to lower intraocular pressure. Glaucoma is often asymptomatic until treated, and the single greatest decline in a glaucoma patient’s quality of life will often occur on the day that treatment is initiated. Because we have come to appreciate the value in preserving quality of life for our patients, I would like to consider issues in selecting glaucoma therapeutic strategies, and for the purposes of this discussion, I will discuss primary medical therapy, though primary laser trabeculoplasty is an option for open-angle glaucoma and ocular hypertensive patients.
Multiple Meds Needed
Adequate IOP control often requires more than one medication. In the Collaborative Initial Glaucoma Treatment Study, 35 percent IOP reduction was achieved on average for glaucoma patients in the “medication arm.” However, a review of the protocol and results will show that about half of those patients in the medication arm could not reach their target IOP on drops and “crossed over” to therapy with argon laser trabeculoplasty. While it is often stated that the visual fields were stable in CIGTS, by nine years, 23.1 percent of the medicine group and 34.1 percent of the surgery group experienced a 3-dB or greater worsening of the visual field.1 Another way of summarizing CIGTS is that many of our glaucoma patients will require multiple medications and some will progress, and this was found to be the case with IOPs consistently below 18 or even 16 mmHg.
Target IOP
The concept of seeking a target IOP or target IOP range has gained a significant level of acceptance in glaucoma management; however, it remains unclear whether practitioners should seek a percentage reduction from baseline, an absolute number (“low teens”), or something else, such as low IOP fluctuation or low nocturnal IOP.
|
Because preserving quality of life is so important, before prescribing IOP-lowering therapy, I ask my patients a simple question: “What do I need to know about you before we pick a medication to lower your eye pressure?” The question is intentionally open-ended and allows the patient to frame a discussion about what medication or approach might be best for him or her. If the patient needs a little prompting, I will ask about medication copays or if she has had problems with side effects from medications in the past. Often, the patient will jump right in with important information. Having asked this question for several years, I have been impressed by the range of responses I have received and by my own inability to predict which patients will first raise the issue of cost and which will be concerned about side effects, etc. I am, of course, most pleased when patients turn the autonomy over to me—“Choose what you think is best.”
In my opinion, the best way to treat glaucoma is to get the patient on medication that he will remember to take, medication that is financially available and effective for him, and medication that is tolerable. This often involves tailoring treatment for each individual patient, as each patient will vary based on the above factors. With the recent availability of generic latanoprost, we now have the entire complement of medical therapeutic classes available in generic form. On a number of occasions, I have had patients insist that I not treat them with generic medications. Why would a patient make this request? We live in a branded world. When was the last time that you were offered a generic beverage at a restaurant or on an airplane? We trend towards brand-name drinks because of the consistency and availability of the product. Are eye drops that different?
Brand-Name vs. Generic
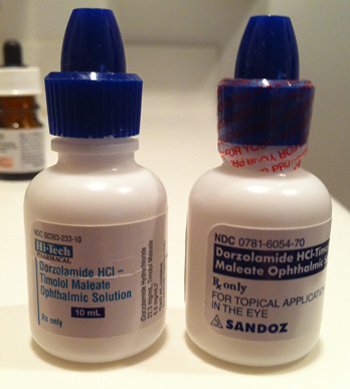
While there is a paucity of data comparing the efficacy of brand-name versus generic IOP-lowering medications, I believe there are compelling benefits of brand-name medications. Consider the case of brand-named Cosopt. Cosopt is a familiar product, and like many brand-name products, it is recognizable in its name, appearance, and even feel (square bottle). Think for a moment what happens when a patient is switched from the branded medication Cosopt to its generic counterpart, dorzolamide hydrochloride 2% timolol maleate 5%. First of all, the name of the patient’s medication becomes seven times longer and infinitely more difficult to pronounce. It is no longer a medication that the average patient will be able to report to an internist without a medication list. When my patients come in to the office and we ask them what medication they are taking, they will often report their generic fixed-combination as timolol or dorzolamide (not both). Because of the generic Cosopt issue, I ask every patient to bring in every drop she is taking. Because of this confusion, I have seen “generic Cosopt” patients who are taking additional timolol medication (thinking they are only on dorzolamide), and vice versa.
|
We have also recently learned the value of ophthalmic formulations. Products such as Alphagan P 0.1% and Lumigan 0.01% are examples of medications that have undergone changes in concentration of the active ingredient, pH, preservative or other excipients to achieve a more favorable safety and tolerability profile.
Several agents are now available without preservatives (Zioptan, preservative-free Cosopt) or with alternative preservatives (Travatan Z). We now understand that an eye drop is more than just a drug in a bottle. There are other components that also contribute to the final product and its safety profile, such as preservatives and the actual characteristics of the bottle. A recent Canadian study evaluated five bottles each of 11 kinds of glaucoma drugs to determine whether brand-name glaucoma drugs differ from generic equivalents in bottle design, viscosity, surface tension, and volume in North America.4 For the American brand-name Timoptic XE, the average drop volume was 38 ±3.1 µL compared with 24 ±1.5 µL of timolol GFS. For the Canadian brand-name Timoptic XE, the average drop volume was 42 ±4.0 µL compared with 25 ±2 µL of timolol maleate EX. The surface tension of the Canadian Timoptic XE was 31 percent higher than that of the generic, and the surface tension of the American Timoptic XE was 21 percent higher than that of the generic. Additionally, the bottle tips of the Canadian and American Timoptic XE measured approximately 3.5 times larger than those of their generics.
Malik Kahook, MD, and colleagues recently compared generic and brand-name versions of latanoprost and fixed-combination dorzolamide-timolol of temperatures of 25° and 50°C for 30 days.5 To begin with, several generic latanoprost bottles contained drug concentrations greater than 10 percent of the labeled concentration. At the higher temperatures, generic and brand-name dorzolamide-timolol were resistant to degradation, but at both 25° and 50°C, generic latanoprost formulations saw significant loss of active ingredients. Finally, particulate matter not seen in brand-name bottles was seen in the bottles of both generic medications.
|
We all have patients who have financial concerns, and glaucoma medications can be expensive. Interestingly, laser trabeculoplasty is not necessarily more cost-effective than medications, particularly prostaglandin analogs. Joshua D. Stein, MD, MS, and colleagues recently evaluated the cost-effectiveness of laser and prostaglandin analogs and found that prostaglandin analogs provide greater health-related quality of life relative to laser trabeculoplasty, assuming excellent compliance with the medications.6
Fixed-combination medications have several potential benefits over the use of separate bottle individual medications.8 These benefits include a reduction in the total number of drops and preservative instilled each day, cost savings, improved tolerability and compliance and avoiding the washout effect resulting from rapid-sequence instillation of multiple drops. Current commercially available fixed-combination drugs include the topical beta-adrenoceptor antagonist timolol 0.5% combined with a prostaglandin, a topical carbonic anhydrase inhibitor or an alpha-adrenoceptor agonist. Fixed-combination drugs are convenient; however, they remove the possibility of titrating the individual components both in terms of concentration and timing of administration. Additionally, they may make it more difficult to determine which of the two components of the medication may be causing an adverse reaction or side effect. For each of our patients, we must weigh the convenience of these medications against their limitations.
I am often asked if I’ve observed loss of IOP control in patients who have been switched from a brand-name to a generic medication, and the answer is, I can’t be sure. In a study of inter-visit IOP fluctuation in untreated ocular hypertensive patients enrolled in the Ocular Hypertension Treatment Study, Anjali Bhorade, MD, and colleagues found that 13 percent of eyes had more than 20 percent change in IOP between consecutive visits. Considering that 20 percent was roughly the efficacy of beta-blocker therapy in the OHTS study, a significant percentage of patients experienced clinically meaningful IOP fluctuations between visits. The authors concluded that intervisit IOP fluctuations were of significant magnitude in some cases to mask or mimic the effects of glaucoma medications.9
|
Maintaining Consistent IOP
Investigations by Robert Weinreb’s lab at the University of California, San Diego found that, even in normal people, IOP is higher at night, particularly when measured in the supine position.10 This is also the case in glaucoma patients, and certain glaucoma medications don’t work quite as well at night; however, the prostaglandin analog class does pretty well controlling the pressure during the nocturnal period, even in the supine position. While it intuitively makes sense to us that we should try to control IOP 24 hours a day, we do not have data that indicate how important nocturnal IOP reduction is in glaucoma management. In addition to IOP, many other variables change at night in the supine position, including cerebrospinal fluid pressure and blood pressure, and the complex interaction of known and unknown factors could affect the importance of lowering IOP at night.
Recent technological developments have increased the likelihood that we will soon have high-quality 24-hour IOP data available in order to critically evaluate the importance of lowering IOP 24 hours per day. Dr. Weinreb recently published an article discussing two approaches being investigated for 24-hour IOP monitoring for patients with glaucoma.10 The first is permanent IOP monitoring through an implantable sensor, and the other is temporary monitoring through a contact lens sensor. Studies suggest that 24-hour continuous IOP monitoring can be integrated into clinical practice and has the potential to contribute to reducing glaucoma-related vision loss.
In summary, the landscape of glaucoma is changing. As new data becomes available, we may soon adjust our IOP-lowering strategies or at least better understand which aspects of IOP to target. Anthony Realini and Robert Fechtner have written about 56,000 different eye-drop combinations that can be used to treat glaucoma, and the potential for complex regimens highlights the need to simplify and streamline glaucoma therapy for our patients.11 In my practice, I discuss treatment goals with my patients and try to determine whether the avoidance of generic therapy makes sense for them, rather than assuming that they would inherently prefer generic medications. Given the likelihood that patients will either require more than one medication or progress even with significant IOP lowering, choosing an effective and well-tolerated initial glaucoma medication is worthy of a thoughtful discussion between the physician and patient. REVIEW
Dr. Radcliffe is an assistant professor of ophthalmology at Weill Cornell Medical College, New York-Presbyterian Hospital, New York City.
1. Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R; CIGTS Study Group. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology 2011;118:1766-1773.
2. Brandt JD, Beiser JA, Gordon MO, et al. Central corneal thickness and measured IOP response to topical ocular hypotensive medication in the Ocular Hypertension Treatment Study. Am J Ophthalmol 2004;138:717-722.
3. Juzych MS, Randhawa S, Shukairy A, Kaushal P, Gupta A, Shalauta N. Functional health literacy in patients with glaucoma in urban settings. Arch Ophthalmol 2008;126:718-724.
4. Mammo ZN, Flanagan JG, James DF, Trope GE. Generic versus brand-name North American topical glaucoma drops. Can J Ophthalmol 2012;47:55-61.
5. Kahook MY, Fechtner RD, Katz LJ, Noecker RJ, Ammar DA. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res 2012;37:101-108.
6. Stein JD, Kim DD, Peck WW, Giannetti SM, Hutton DW. Cost-effectiveness of medications compared with laser trabeculoplasty in patients with newly diagnosed open-angle glaucoma. Arch Ophthalmol 2012;130:497-505.
7. Rylander NR, Vold SD. Cost analysis of glaucoma medications. Am J Ophthalmol 2008;145:106-113.
8. Khouri AS, Realini T, Fechtner RD. Use of fixed-dose combination drugs for the treatment of glaucoma. Drugs Aging 2007;24(12):1007-1016.
9. Bhorade AM, Gordon MO, Wilson B, Weinreb RN, Kass MA; Ocular Hypertension Treatment Study Group. Variability of intraocular pressure measurements in observation participants in the ocular hypertension treatment study. Ophthalmology 2009;116:717-724.
10. Mansouri K, Weinreb R. Continuous 24-hour intraocular pressure monitoring for glaucoma—time for a paradigm change. Swiss Med Wkly 2012;142:w13545.
11. Realini T, Fechtner RD. 56,000 ways to treat glaucoma. Ophthalmology 2002;109(11):1955-1956.