For centuries, we clinicians have recognized that ocular hyperemia is a hallmark of several ophthalmic pathologies. At the same time, however, we may have been disregarding the importance of the characteristics of the redness itself. In this article, we'll break down redness into its components and help you determine what they mean in terms of the health of the eye.
Anatomy and Physiology of Hyperemia
While the avascular cornea, particularly the peripheral region, acquires its nutrients from many sources, such as the aqueous humor and the tear film, the conjunctiva and anterior episclera are nourished by vessels from the anterior and long posterior ciliary arteries, which stem from the ophthalmic artery. As with any inflammatory reaction to a tissue insult, patterns of dilation in this vasculature result in hallmark signs of inflammatory responses. Vasodilation—the increased diameter of blood vessels—can lead to increased blood flow and leakage of proteins and fluid from the capillaries, which can result in edema, increased inflammatory cells and mediators, and tissue damage. All of these factors contribute to the characteristics of hyperemia on the ocular surface. Vasodilation itself can be caused by vasoactive amines such as histamine, hypoxia and a full array of other factors that induce endothelial cell spreading and flattening and the opening of capillary sphincters, allowing for leakage of proteins and fluid.
Redness of the conjunctival, episcleral and ciliary vessel beds is known to vary in color, location and degree with different diseases and maladies. Generally, the location of the vasodilation, the hue (depth of color) and the intensity of the redness are important features that help to classify the hyperemia and make the diagnosis.1 In terms of location, there are the meandering conjunctival vessels that tend to be superficial in the angular, bulbar, palpebral and/or limbal regions; the fine, linear, deeper episcleral vessels; the deepest, large vessels of the sclera; and the vasculature of the lid.
Deciphering Redness
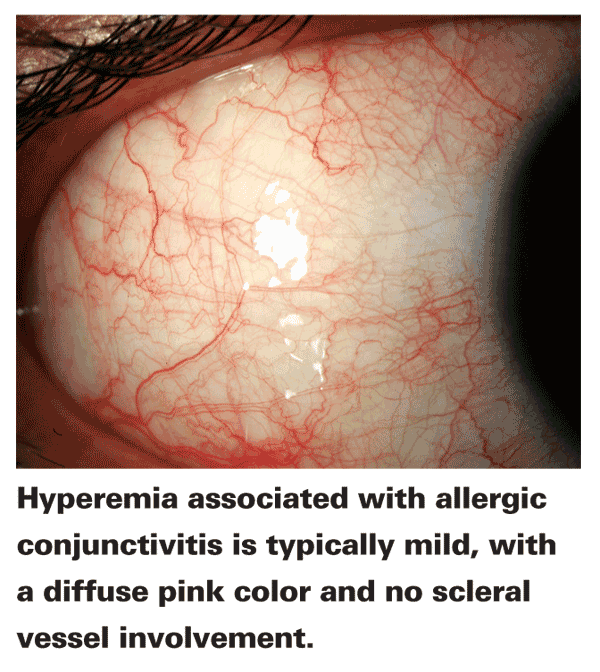
• Allergic conjunctivitis. Hyperemia associated with allergic conjunctivitis is typically a mild, diffuse pink with no scleral vessel involvement. This redness results from vasoactive amine activity, specifically histamine binding to H1 and H2 receptors on the vascular conjunctival endothelium, which causes the thinning of the endothelium and gaping of the capillaries, allowing for vasodilation.2 The muted color intensity is a result of overlying conjunctival chemosis in ocular tissue: As fluid leaks out of the capillaries, it creates a hazy effect over the vessels, resulting in an apparent pink color.3
• Morning eye congestion. When allergens, microorganisms and irritants become caught in the tear film beneath the lids during sleep, a mild state of inflammation can result. While conjunctival leukocytes respond in an effort to eliminate these intruders, meibomian and lacrimal secretions continue to introduce tear ingredients including lactoferrin, lysozyme, lipocalin and IgA, which contribute to the innate ocular defense.4 The lid edema and hyperemia typical of inflammation can be observed upon waking as the appearance of puffy lids, also known as morning eye congestion (Breton, et al. IOVS 2010;51:ARVO E-abstract #2389).5
• Dry eye. The causes of dry eye vary, and include environmental exposure (e.g., low humidity), dehydration, nutritional factors, activities (e.g., visual tasking), chronic systemic disease, use of systemic medications with anticholinergic effects and hormonal changes. While the underlying etiology of dry eye can be difficult to determine on a case-by-case basis, the ocular hyperemia appearing due to the condition is fairly consistent. Fine horizontal vessel dilation, mainly in the interpalpebral fissure, is common. Generally the hyperemia indicative of dry eye has a mild red shade. If a clinician observes this variety of redness, he should pursue other dry-eye diagnostics (e.g., tear-film breakup time, Schirmer's testing, Ocular Protection Index, ocular surface staining via fluorescein or rose bengal dyes, symptomatic breakup time, tear meniscus height, etc.). Asking the patient about any symptoms of burning, stinging, dryness and grittiness, among others, can further support diagnosis.
• Contact lenses. When the root of the diagnosis relates to contact lens wear, a circumlimbal presentation of hyperemia is typical. The intensity and amount of redness present tends to vary in conjunction with the degree of corneal edema and/or associated iritis. A circumlimbal pattern should lead the practitioner to carefully examine the fit of the lenses and evaluate the potential for changes in wear regimen or lens design.
• Ocular drug-induced allergic reaction. A redness in the lower half of the eye is often a sign of allergic reaction to a topical ophthalmic agent. The lower half of the conjunctiva along with the inferior cul-de-sac and lower lid generally demonstrate substantial levels of redness, while the upper counterparts remain clear due to the gravitational and dilution effects on the residence of ophthalmic solutions. When a patient presents with hyperemia matching this description suggesting a drug-induced ocular allergic reaction, the clinician's first attempt at remedying the redness should be to remove suspected agents and adjust treatment plans accordingly.
• Marginal infiltrates of the cornea. Marginal infiltrates describes the condition resulting from leukocytes leaking into the peripheral cornea. Blood vessels extending from the deep episcleral and anterior conjunctival arteries reach only about 0.5 mm into the cornea, but marginal infiltrates can appear 1 to 2 mm in from the limbus if leukocytes leak out of these vessels into the cornea.6 Typically, the infiltrates are accompanied by fine vessel dilation and localized, quadrantic, deep pink, conjunctival redness. Observation of these characteristics suggests the need for a thorough clinical assessment of the entire limbal region for infiltrates.
• Bacterial conjunctivitis. Superficial vasodilation, especially when it appears in the bulbar conjunctiva, is often indicative of bacterial conjunctivitis. Redness is rarely present in the limbus. Generally, the redness will manifest unilaterally, at least at the start of the infection, and will progress in intensity over time. While the infection is generally self-limiting, prevention of spread and sequelae is important.
It's also difficult to differentiate bacterial conjunctivitis from viral conjunctivitis in the early days before follicles or pretragal nodes develop. The presence of mucopurulent discharge can corroborate the diagnosis, but bacteriologic culture is the ultimate test for determining the ideal treatment for these cases.1
• Severe ocular infection. Serious, sight-threatening infections including endophthalmitis and corneal ulceration also demonstrate distinctions in terms of their patterns of redness. The appearance of an intense "fire engine" red or near-purple hue, often with deep scleral vessel involvement, is cause for concern and further examination. The practitioner should perform a culture to determine the microbial organism at fault, and pursue aggressive broad-spectrum treatment until causation is confirmed.
• Iritis. Although varied in pathological origin, careful diagnosis and treatment of iritis is essential to avoiding sequelae such as cataract and glaucoma.
A pink, halo-like circumlimbal flush of dilated ciliary blood vessels is a hallmark of iritis. Observation of this characteristic flush should prompt clinical consideration of anterior uveitis and further evaluation to identify the root cause, in order to implement the proper treatment approach.
How Red?
Approaches to quantifying and classifying the extent and quality of ocular redness have varied over the years. Here's a look at how to approach this task.
• The portrayal. Numerical scales are the mainstay used by most practitioners and clinical researchers, but the existing numerical schemes and pictorial representations differ greatly. Some scales are based on varied verbal description and others on pictorial or photographic representations, while some others integrate numerical designations with verbal and photographic representations. The range of numbers presented varies by scale as well, with some scales ranging from 0 to 4 and others ranging from 0 to 100. Some scales are discrete while others are continuous. Research demonstrates that practitioners tend to use a limited selection of numbers (e.g., using intervals of 5 or 10 on a scale ranging from 0 to 100 and using whole or half number increments on continuous scales from 0 to 5).7,8 This finding may suggest the utility of discrete scales for evaluation.
• The interpretation. One of the most important aspects of redness scales is the interpretation of the representations, whatever their form. In redness scales, one of the pervasive considerations is the "normal" state of redness (generally depicted as a zero or the lowest number on a numerical scale). What a "zero redness" state looks like in one patient is likely different from that of another patient. Vasculature will also vary slightly among patients, and certain individuals demonstrate some structurally non-responsive vessels (i.e., vessels that do not demonstrate vasodilation or vasoconstriction). These factors can further promote differences between the depiction of a score on the scale and that observed through the slit lamp, as well as baseline variables. Environmental factors like wind, smoke, eyestrain, dust and humidity can also impact redness, even in healthy eyes.9,10 One study of 121 normals (with "white" eyes) demonstrated an average bulbar redness of 1.9 units using the Cornea and Contact Lens Research Unit scale.10 This is one area for consideration both in the design and application of grading scales.
Methods to minimize inter-observer variation resulting from the use include: multiple observer grading for comparison of results; specific clinician training; and psychophysical scale development using clinician input.8 Others suggest that a personalized normalization of scales for each individual observer may be helpful in standardizing scale interpretation. Similar to a golf handicap, graders would apply a "correction factor" to their grade of the redness, standardizing inter-observer error.7
• The future. Along with the progress in disease-specific scales, other attempts to quantify redness include image processing analysis based on the percent of redness color and the fraction of pixels representing blood vessels, and forays into fractal analysis, which incorporates the degree of branching in the vascular map of the eye.11-13 Researchers have used the fractal analysis technique to increase the objectivity of bulbar conjunctival redness scales.
Clearly, appreciation for the ocular surface characteristics has value in two areas: First, it provides additional diagnostic clues to the clinician; and second, it lets us refine the development of validated scales to provide a central basis for drugs acting on the ocular surface, either by pharmacologically producing or decreasing redness directly or by reducing the underlying causes of the redness. Ideally, classification of redness would be performed by a panel of experienced and trained clinicians with reference to scales designed appropriately for the specific condition and with knowledge of the baseline patient status. In short, attending to the vessel location, hue and intensity of ocular hyperemia can be a critical tool in the hands of external disease diagnosticians.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Terry RL. Clinical recognition of anterior segment inflammatory disease. In: Stapleton F, ed. The Anterior Eye and Therapeutics: Diagnosis and management.
2. Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge. A clinical approach to studying allergic conjunctivitis. Arch Ophthalmol 1990;108:1:84-8.
3. Abelson MB, Pyun J. Histamine. In: Allergic Diseases of the Eye.
4. Sack RA, Conradi L, Krumholz D, et al. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: Angiogenin and other defense system constituents. Invest Ophthalmol Vis Sci 2005;46:4:1228-38.
5. Sack R, Conradi L, Beaton A, et al. Antibody array characterization of inflammatory mediators in allergic and normal tears in the open and closed eye environments. Exp Eye Res 2007;85:4:528-38.
6. Abelson MB, Slugg AP. Marginal infiltrates in the cornea. In:
7. Efron N, Morgan PB, Katsara SS. Validation of grading scales for contact lens complications. Ophthalmic Physiol Opt 2001;21:1:17-29.
8. Schulze MM, Jones DA, Simpson TL. The development of validated bulbar redness grading scales. Optom Vis Sci 2007;84:10:976-83.
9. McMonnies CW, Ho A. Conjunctival hyperaemia in non-contact lens wearers. Acta Ophthalmol (Copenh) 1991;69:6:799-801.
10. Murphy PJ, Lau JS, Sim MM, Woods RL. How red is a white eye? Clinical grading of normal conjunctival hyperaemia. Eye (Lond) 2007;21:5:633-8.
11.
12. Schulze MM, Hutchings N, Simpson TL. The use of fractal analysis and photometry to estimate the accuracy of bulbar redness grading scales. Invest Ophthalmol Vis Sci 2008;49:4:1398-406.
13. Willingham FF, Cohen KL, Coggins JM, et al. Automatic quantitative measurement of ocular hyperemia. Curr Eye Res 1995;14:12:1101-8.



