Combining a cataract operation with a glaucoma procedure makes sense for a lot of patients, and the options for doing so are expanding each year. Now, surgeons can choose from less invasive ab interno options as well as traditionally more invasive surgeries that go through the conjunctiva and sclera to reach the anterior chamber. To help keep you up-to-date on what’s out there now and what’s on the horizon, here is a look at procedures throughout the spectrum.
Ab Interno Approaches
Surgical devices and approaches that can use the phaco incision appeal to many surgeons who need moderate pressure lowering and also don’t want a procedure that’s too invasive.
• Glaukos iStent. Fayetteville, Ark., surgeon Steven Vold has worked with the iStent, the data for which is currently under review at the Food and Drug Adminstration. “It’s a small snorkel made of heparin-coated titanium,” he explains. “Through an ab interno approach, you place it right into Schlemm’s canal. In the procedure, you basically tilt the device about 45 degrees, enter the trabecular meshwork and then gently slide it into place. The little snorkel will be in the anterior chamber, and the rim of the device will be stenting open
Schlemm’s canal, allowing egress of fluid through the device. You should place these devices in the nasal quadrant right next to collector channels if you possibly can. The procedure can be done through a very small incision—1 to 2 mm is what we commonly use—or it can be done through the cataract wound.” In the U.S. study, the iStent is used for patients with mild to moderate glaucoma.
|
In the future, if the iStent is approved, it’s possible that surgeons may place multiple stents to achieve even lower intraocular pressures. Six-month data from a prospective, randomized study by the Minimally Invasive Glaucoma Surgery Study Group, presented by Kerry Solomon, MD, at the American Society of Cataract and Refractive Surgery meeting, found an increased effect from more than one stent. Thirty subjects were available for six-month review. The average baseline IOP (after a medication washout) was 24.9 mmHg in groups receiving one or two stents and 24.5 mmHg in a three-stent group. At six months, mean IOP was 16.1 mmHg in the one-stent group, 13.7 mmHg in the two-stent group and 13.6 mmHg in the three-stent group. Medications were reduced from two preop to one postop. At one day, one of the one-stent subjects had transient hypotony and one of the three-stent patients had a small hyphema.
• Endocyclophotocoagulation. In ECP, the surgeon uses an 810-nm laser, aimed via an endoscopic video connection, to ablate a certain number of the ciliary processes to decrease aqueous production. It’s unique in that it’s the only procedure that works by decreasing the production of aqueous rather than increasing its outflow.
Brian Francis, MD, associate professor of ophthalmology at the University of Southern California’s Keck School of Medicine/Doheny Eye Institute, has used ECP in many cases. “It can be used at any point in the treatment spectrum,” he says. “We’ve used it as first-line treatment as well as with trabeculectomy for glaucoma that hasn’t been controlled. However, many of the patients treated with combined cataract and ECP surgery are in the mild to moderate glaucoma category and are controlled with medication.
“There are two ways to perform ECP with cataract surgery,” Dr. Francis continues. “Both of them go through a clear corneal incision. In the first method, if you’re going to ablate 270 to 300 degrees of the ciliary processes, you can enter through the clear corneal incision. In the second, if you want more pressure reduction, you do a 360-degree treatment, which involves also going through the paracentesis, which you can enlarge to do it.” Dr. Francis says to expect about a 30-percent reduction in IOP from baseline, depending on the patient’s baseline IOP and how aggressive the ECP treatment is. “In the mild to moderate glaucoma group, you may see less of a pressure reduction,” he says. “However, you’ll see a reduction in medications. The medication reduction can be between one and two drugs.” Dr. Francis says phaco and ECP work well together because they’re complementary methods of pressure reduction, rather than redundant approaches that work by the same mechanism.
• Transcend Medical’s CyPass. The CyPass is a tubular micro-shunt composed of biocompatible polyamide material. It’s implanted in the suprachoroidal space, between the sclera and the ciliary body, to help increase aqueous outflow.
Dr. Vold has been working with the CyPass since the fall of 2009 as part of a trial of the device, and has implanted it in just over 60 patients. “The population for the CyPass is people with cataracts and mild to moderate glaucoma,” he says. “In our patients, we’ve found that it yields 30 to 40 percent IOP lowering early on, and we’ve been able to get a vast majority of them off their glaucoma medications.
“Again, these are mild to moderate glaucoma patients, so it’s possible that they can scar down over time,” adds Dr. Vold. “And there’s no question that some of them ultimately will. So far, though, most of these patients have done well.”
The CyPass implantation procedure is similar to that used for the iStent. “We do the cataract surgery first,” explains Dr. Vold. “Then, using a direct gonio lens, I slide this into the suprachoroidal space at the base of the scleral spur. There are a couple of surface features on the shunt that allow it to be positioned so the head of the CyPass device is just in the anterior chamber. I like to have it placed between the trabecular meshwork and Schwalbe’s line. This way, you don’t have to worry about occlusion of the device, yet you also don’t have to worry about damage to the corneal endothelium.”
To help make gonioscopic surgery easier, Dr. Vold has developed a goniolens with Transcend Medical that will hold the eye in place and ensure that the surgeon doesn’t have to tilt the microscope. “That tilting of the microscope becomes an issue for a lot of people,” he says. (Dr. Vold is a consultant to Transcend, but doesn’t receive royalties for the lens.)
In terms of complications, Dr. Vold says that, similar to the iStent, bleeding is something to be mindful of, as is inadvertent movement of the device. None of his have moved, though. “There’s also a potential risk for hypotony,” he says, “since you’re essentially forming a cyclodialysis cleft with the device, but this hasn’t been an issue in the study.”
• NeoMedix Trabectome. The Trabectome uses bipolar cautery on a disposable handpiece to ablate a certain number of clock hours of trabecular meshwork in an effort to increase outflow. The handpiece also has irrigation and aspiration ports.
|
In practice, he says the procedure can be done before or after the phaco, and each approach has its own advantages. “If you do the Trabectome first, you have the best view of the angle, since the eye is pristine and there is no corneal opacity from the thermal effects of phaco,” he explains. “Also, the Trabectome incision is smaller than that used for phaco, so if you make the Trabectome incision first, you will get a nice seal. You can then enlarge that incision afterward to do the phaco. The advantage of doing the phaco first is that the patient will be dilated. You remove the lens and can then put in Miochol to constrict the pupil, which makes the Trabectome a little easier.”
Nils Loewen, MD, PhD, of the University of Pittsburgh Glaucoma Service, says that when using the Trabectome before phaco, surgeons can take certain steps to enhance outcomes: “If you push outward as you use the Trabectome, you can ruin all the collector channels,” he warns. “Instead, you almost have to pull in as you treat, or at least not push outward. Also, for visualization, rotate the goniolens 90 degrees and then tilt it toward either the brow or the cheek, depending on where you’re treating, and you will be able to see farther. Also, when you’re retracting from the eye, tamponade the treated area with DisCoVisc.”
• Ivantis Hydrus. The Hydrus is a device made of nitinol that can be implanted through the cataract wound. It goes into Schlemm’s canal, covering a large area—three clock hours—stenting open the standard outflow channels. Its U.S. clinical study began in February 2012.
Dr. Vold has worked with the device and says its implanted location is similar to the iStent’s, but the device itself is larger. “It’s a bigger device than the others,” he says. “The early data on it has been very good, and we’ve gotten pressures consistently into the low- to mid-teens, with patients recovering quickly after surgery. As a bigger device, one of the issues you might expect is erosion. However, after several years of use overseas only one has had to be explanted, and patients have done well with it. There’s probably a little more surgical skill involved with implanting it compared to some other devices, but once you learn how to do it, it’s definitely something that’s doable for a good cataract surgeon.” The device is mostly being used for open-angle patients, with mild to moderate glaucoma. “Prior argon laser trabeculoplasty is a contraindication,” says Dr. Vold. “But prior selective laser trabeculoplasty patients should be all right.”
• The AqueSys device. This device is a gelatin tube that uses an ab interno approach to reach an area usually accessed via more invasive ab externo methods: the subconjunctival space. It has recently begun its trial for 510(k) clearance in the United States. Though it could be done as part of a cataract operation, in the U.S. trial it’s being studied as a stand-alone procedure in refractory glaucoma.
“With the AqueSys device, you leave a small portion of it in the anterior chamber, about 3 mm within the sclera and a couple of millimeters within the subconjunctival space,” explains Dr. Vold, who is taking part in the U.S. study. “The proposed advantage is that it may be much less traumatic than a trabeculectomy or an ExPRESS mini-shunt, yet we can get low lying blebs with it. Right now one of the questions is whether or not we should use an antimetabolite with it, though we’re not using one in the studies. Also, when you create an opening like this, there are always the concerns of early hypotony, and possibly dealing with the risks associated with that. However, the safety profile from overseas has been good thus far. In terms of results, the early results here show pressures going from the low to mid-20s to the mid-teens.”
• Excimer laser trabeculostomy. ELT uses fiber-optically delivered 308-nm ultraviolet energy to ablate juxtacanalicular trabecular meshwork and the inner wall of Schlemm’s canal in an effort to create openings that stay open over time.
Beverly Hills, Calif., glaucoma specialist Michael Berlin developed the device and the procedure, which can be used through the existing cataract incision. “The goal in the future would be to perform a high-resolution OCT canalogram to find out where the shortest distance is to the larger collector channels and place the ELT openings in that region to maximize the outflow,” he says. “But until we have that technology, we have to approximate, with most of the openings now being placed in the infero-nasal quadrant.” The procedure usually makes 10 spots in the meshwork, which results in five openings into Schlemm’s canal to help increase outflow.
In a small, prospective study, 37 eyes of 37 patients with an average preop IOP of 23.3 mmHg received ELT in conjunction with cataract surgery. At five years, the mean IOP is 14.3 mmHg, and the average number of meds decreased from 1.4 to zero. (Giers UF, et al. IOVS 2012;53:ARVO E-Abstract 5957)
The ELT procedure is currently CE-marked, but isn’t FDA approved. “I’m hoping to be able to start a study soon, within the next six to 12 months,” says Dr. Berlin. “This will add data for an FDA approval.”
Ab Externo Approaches
For certain cases, surgeons may turn to procedures that are considered more invasive. Here is a look at three, including one that’s not approved in the United States.
• Alcon’s ExPRESS Shunt. The ExPRESS mini-shunt is placed under a scleral flap, with one end in the anterior chamber and the other beneath the flap, in a procedure similar to conventional trabeculectomy.
|
“Regarding ExPRESS implantation, I think it’s important to make the incision parallel to the iris before placing the ExPRESS and to not make it too anterior,” Dr. Sarkisian continues. “You don’t want the ExPRESS sitting in clear cornea instead of in sclera. I like to visualize the line between cornea and sclera—the ‘blue line’—and make my wound just behind it.”
In terms of results, one long-term study of 39 eyes found that, at three years, two-thirds of ExPRESS cases had IOPs of 18 mmHg or lower with no need for medications.1
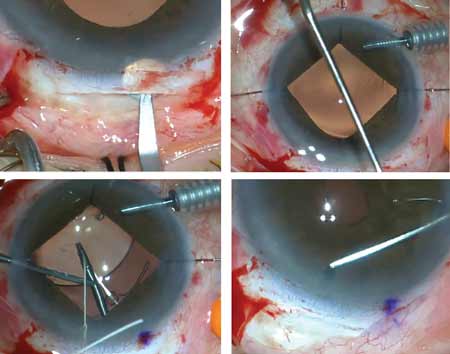
• Canaloplasty with the iScience microcatheter. Canaloplasty is a procedure that aims to reduce IOP in open-angle glaucoma patients by circumferentially viscodilating and tensioning the inner wall of Schlemm’s canal.
Bradford Shingleton, MD, associate clinical professor of ophthalmology at Harvard Medical School, performs a range of glaucoma procedures, one of which is canaloplasty. “I find canaloplasty is an effective procedure for patients who need greater pressure reduction but in whom we don’t want to deal with bleb formation,” he says. “That’s an important consideration. The pressure reduction doesn’t tend to be quite as low as with trabeculectomy, but it’s much greater than cataract surgery alone or the phaco/minimally invasive glaucoma surgeries.
“When combining canaloplasty with cataract surgery, I do the cataract operation first on the temporal aspect and place a suture in the keratome incision,” Dr. Shingleton continues. “I even place a suture in the paracentesis incision so we’re able to manipulate the eye for the canaloplasty. I then shift superiorly after completion of the cataract operation to do the canaloplasty. I favor a fornix-based flap, and we do our superficial scleral flap of approximately half scleral thickness. We then do a deep scleral flap, and the key is to start posteriorly enough to be able to move forward to unroof Schlemm’s canal. The key thing for mobilization of the deep scleral flap is to be very deep; you want to be just above, but not enter, the suprachoroidal space. As you mobilize forward you’ll be able to unroof Schlemm’s canal. Then we carry the deep sclerectomy flap forward to create a clear Descemet’s window. We then viscodilate Schlemm’s canal and place the iScience microcatheter in the canal, guided by the HeNe beam to visualize the canal. Generally, I find that passage of the cannula is quite easy.
“Occasionally, there can be obstruction with the collector channels. If that’s the case, you come out and go around the opposite direction with the microcatheter,” says Dr. Shingleton. “More than 80 percent of the time, we can cannulate Schlemm’s canal 360 degrees. We then tie a prolene suture around the iScience microcatheter. The catheter is brought back around as we viscodilate Schlemm’s canal and the suture is intubated into the canal. The suture is tied down tightly underneath the deep scleral flap. Then we amputate the deep scleral flap anteriorly to create a space that we call the scleral lake. That space will be between the deep scleral bed and superficial flap that’s closed tightly with sutures. We strive for tight closure. The conjunctiva is then closed, again with the goal of not having aqueous egress underneath the scleral flap, to avoid formation of a bleb.”
• Solx Gold Shunt. The gold shunt is usually implanted through a scleral approach into the suprachoroidal space in an effort to enhance outflow. It’s not approved in the United States, and is currently in a 510(k) trial for patients with refractory glaucoma.
“With any of these suprachoroidal devices, they tend to scar down,” says Dr. Vold, who is taking part in the device’s study. “And in the FDA clinical trial evaluating refractory glaucomas, you’re talking about patients who have already failed glaucoma surgery. These are challenging patients to treat successfully.” He says the study is nearing its final results, though it will be at least a year to 18 months before the data is ready for the FDA’s review. REVIEW
1. de Jong L, Lafuma A, Aguadé AS, Berdeaux G. Five-year extension of a clinical trial comparing the EX-PRESS glaucoma filtration device and trabeculectomy in POAG. Clin Ophthalmol 2011;5:527-33.