Replacement of cataractous lens has become the most frequently performed surgery in the world. Yet until recently, not much has been known about the process taking place as a cataract forms, or why cataracts—especially nuclear cataracts—form at different times in different individuals' eyes.
Two approaches have traditionally been used to detect and follow cataract development: subjective lens observation using slit lamp microscopy, and measuring lens transparency or opacity using Scheimpflug technology and retroillumination. Thanks to new technology and a breakthrough in our understanding of cataract formation, two new approaches now look promising. The first uses laser light scattering technology to detect changes in the lens long before light transmission through the lens is significantly diminished, which may allow surgeons to determine how close a patient is to developing a cataract. (This technology can also detect proteins in the lens that indicate early Alzheimer's disease.) The second approach promises to provide a much more precise way to quantify the opacification of the lens as a cataract develops, using anterior segment optical coherence tomography. National Institute of Health researcher Manuel Datiles, MD, and NASA scientist Rafat Ansari, PhD, have pioneered the development of the former; researchers working in Hong Kong and the
Proteins and DLS
The first technology uses dynamic light scattering (sometimes referred to as "photon correlation spectroscopy" or "quasi-elastic light scattering") to analyze the protein composition of a substance such as a human crystalline lens. Dr. Datiles explains how this approach to cataract detection works, and the fortuitous circumstances that brought him together with Dr. Ansari at the right moment to develop it.
"Most current instruments for detecting cataract in the crystalline lens use photography or imaging to describe and grade changes in the lens by measuring the amount of light that's scattered back toward the film or sensor," explains Dr. Datiles. "DLS is completely different; this technology evaluates the protein content of the lens; it's not based on photography.
"Every type of molecular particle has a signature Brownian movement —some move fast, some slowly," he continues. "This instrument projects low-power laser light into a target and measures how the light is scattered by the movement of the molecules over a period of five seconds. That tells us the size of the particles and allows us to determine the identity and quantity of the proteins being scanned. Thus, we can monitor the protein composition of the lens."
Dr. Datiles was a member of a team at NEI that was working on finding a cure for cataracts. "My particular assignment was to find ways to detect cataracts early," he says. "The earlier we can detect cataracts, the earlier we can intervene to prevent the cascade of causative events before it becomes unstoppable."
Dr. Datiles says that he was aware of dynamic light scattering technology and its ability to analyze protein content. "Some groups had previously tried to use this technology to analyze the crystalline lens," he notes. "Researchers at MIT and the
"It happened that Dr. Ansari was developing and miniaturizing this technology for use on the International Space Station," he continues. "He was using it to observe crystallization in space, where the zero-gravity environment allows protein crystals to grow larger and more perfectly than on Earth. Then, his father developed cataracts.
When Dr. Ansari started reading the literature on cataract development, he decided to see whether this technology could analyze the proteins in a crystalline lens.
Experimenting with bovine eyes, he realized that it could. And because his instrument was miniaturized and used low-power lasers, it could easily be mounted on available ophthalmic instruments and used to scan patients."
The Alpha-Crystallin Factor
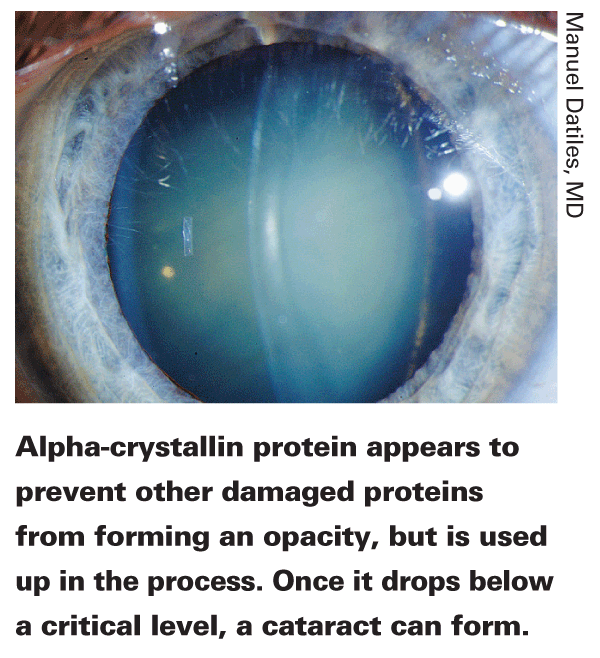
Meanwhile, another breakthrough occurred that made this development even more meaningful. "The proteins in the lens are very specialized," explains Dr. Datiles. "There are three main types: alpha, beta and gamma crystallins. We knew that these proteins clump together into large aggregates and form cataracts, and we knew that alpha-crystallin protein gradually seems to disappear from the lens as people age. But no one knew what alpha-crystallin's special purpose in the lens was, so everyone working on DLS largely ignored it.
"We used reverse psychology," he continues. "If the alpha-crystallin disappears, maybe we should concentrate on it and find out why. Some researchers began to suspect that it was a molecular chaperone, whose job was to prevent other proteins from aggregating.
That would explain why it gradually disappears—and it would give us a way to gauge when a cataract might be about to start forming.
"When beta- and gamma-crystallins and other cellular proteins in the lens are damaged by oxidative stress, they unravel from the tip," he says. "They become sticky and begin attaching themselves to other proteins. Alpha-crystallin, it turns out, plugs up the unraveling tips and prevents the molecules from aggregating further. As a result, the lens remains stable and remains clear. The catch is that we're born with a finite amount of alpha-crystallin, and this process incorporates some of it. So, we gradually use it up as our eyes are subjected to oxidative stress. Eventually, your alpha-crystallin is gone and you have no more protection against cataract development."

Meanwhile, Dr. Ansari's realization that his miniaturized DLS device could measure proteins in the lens eventually led him to contact Dr. Datiles. "We're both working in government, and that makes it fairly easy to collaborate," Dr. Datiles notes. "Dr. Ansari explained that he had created a miniaturized version of the DLS technology that could be mounted on any device we wanted. So, with the help of NASA and NEI administrators, we set up a budget and began working on it."
Dr. Datiles reiterates that timing made all the difference in developing this approach.
"The beauty of the discovery about the purpose of alpha-crystallin was that it came along just when we finally had a device that could detect and measure this protein in vivo," he says. "Now we can measure the amount of alpha-crystallin remaining in a person's crystalline lens, and that can tell us when the lens is in danger of forming a cataract. In fact, we don't have to wait for the cataract to interfere with light transmission to detect and measure it."
Following the Trail
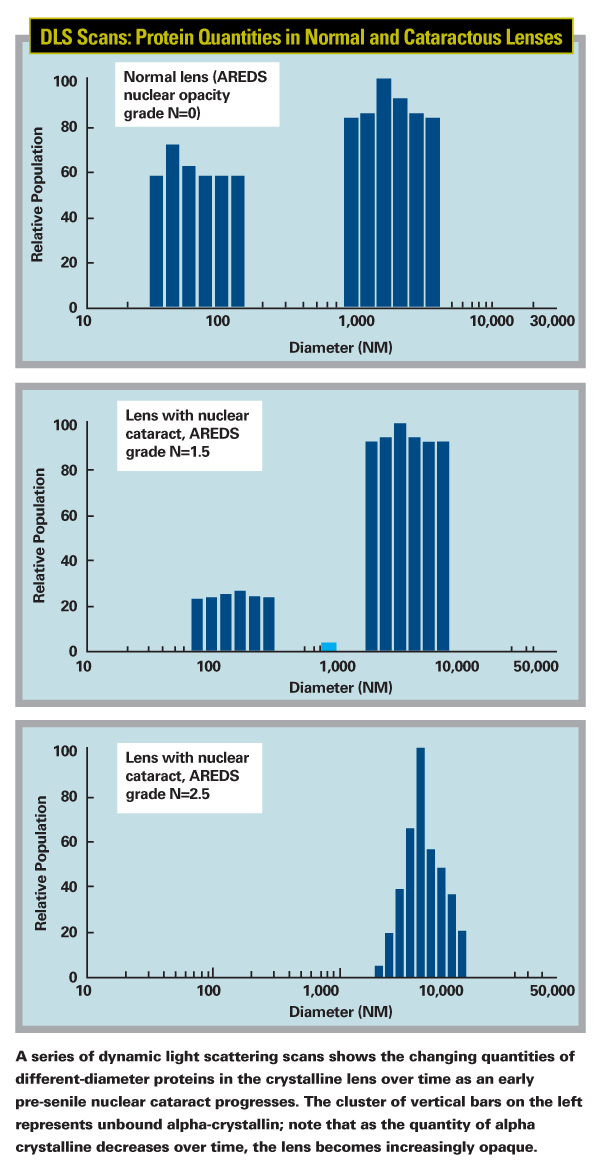
"We quickly realized that this technology was better at detecting cataractous changes than my Scheimpflug instrument, so we looked for a device to mount it on that had a 3-D aiming system," Dr. Datiles continues. "After a lot of trial and error, we finally came up with our current device. We were delighted to find that with the 3-D aiming system we could scan the exact same location at different times. Even more important, with this device we were able to measure the protein makeup of a lens in vivo." The device confirmed the function of alpha-crystallin. "We consistently found that subjects with clear lenses had a lot of alpha-crystallin, while those with cataracts had none," he says.
Dr. Datiles points out that the current instrument's 3-D aiming capability also allows them to sample different regions of the lens. "We usually look at the nuclear part of the lens," he explains. "We take three measurements at a time, to be sure there's no variation between measurements. And while we can monitor a lens over time, a single measurement of the alpha-crystallin is sufficient to give us an idea of what condition the lens is in." Dr. Datiles also notes that alpha-crystallin is a molecular chaperone in other parts of the body as well. "It has the same function—protecting other proteins," he says.
"This suggests the potential to use this technology to monitor lens protein damage as a surrogate for oxidative stress protein damage in other parts of the body."
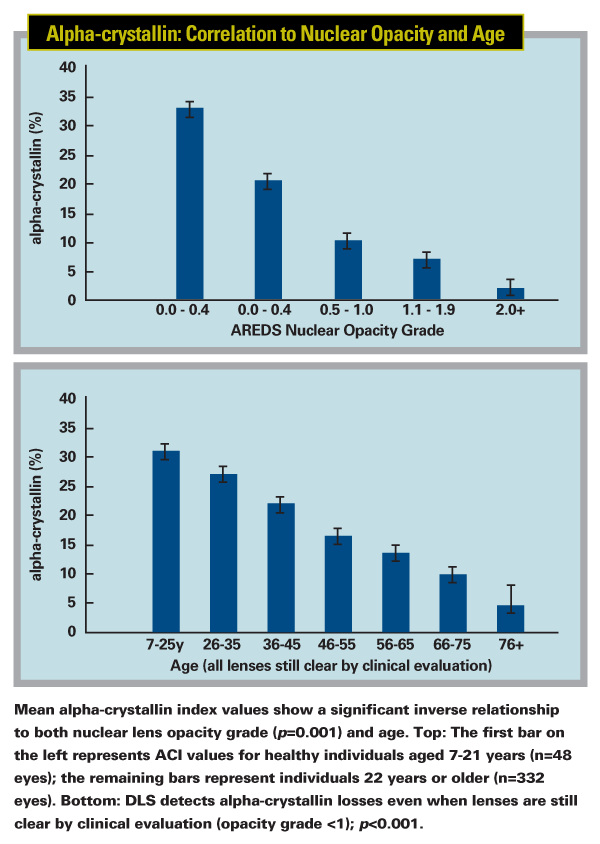
Once they had a working device, they conducted a study on young and old, and clear and opaque lenses to test it; the results were published last year.1 "From our cross-sectional study, we know that young people tend to have a lot of alpha-crystallin," says Dr. Datiles. "However, even in young people we found a variation in the amount. This leads us to wonder about the genetic aspect of this; some people may produce more alpha-crystallin when they're young. This might account for a genetic predisposition for cataract; maybe some people only have 25 percent as much as others do.
"If that turns out to be the case," he continues, "it may be less important how much alpha-crystallin you've lost; the most important thing may be how much you have left.
As long as you still have some left, you're still able to protect yourself from oxidative stress. But once you have almost none, sickness or oxidative stress could be enough to damage your proteins and begin the clumping process. In the animal studies, once the alpha-crystallin is gone and the process starts to develop, it becomes a cascade no matter what you do. We like to think of it as similar to creatinine clearance in kidneys; if you get below a certain level, you start to have kidney failure."
What about other types of cataract? "For now, we're focusing on nuclear cataracts," says Dr. Datiles. "Cortical cataracts appear to be more of a metabolic and osmotic problem than a protein problem. In cortical cataracts you see isolated spokes, which we believe are cause by localized changes—very different from the causes of nuclear cataracts. We haven't done a systematic study of cortical cataracts using DLS, but we assume it can detect those protein changes early as well."
Given that running out of alpha-crystallin allows cataracts to form, why not simply inject more into the lens? "The challenge is getting it through the cornea and into the lens," he explains. "And how do you get the lens to absorb it? For now, when it comes to prevention we're focusing on the alternative to increasing the alpha-crystallin—which is to protect the lens proteins from oxidative and other stresses in the first place."
Multiple Applications
There appear to be many potential real-world applications for monitoring alpha-crystallin:
• Detecting early-stage Alzheimer's disease. "In Alzheimer's, amyloid proteins are deposited in the brain," Dr. Datiles explains. "A researcher at NEI studying animal models of Alzheimer's discovered that these proteins were also appearing in the crystalline lens. Then, a group at Harvard studied post-mortem eyes from Alzheimer's patients and found the proteins deposited between the cortex and nucleus of the lens.
"Dr. Ansari was invited to test the lenses of transgenic mice with Alzheimer's using the laboratory version of the device, and he was able to detect protein changes," he continues. "As a result, we're now doing a pilot study with the DLS device as an early detector for Alzheimer's. If all goes well, this could have a tremendous impact on treatment of the disease; we have drugs that can slow down its progress, so the earlier we can detect the amyloid protein, the more effective those drugs should be."
• Shortening the length of clinical trials. Another use for a device like this is as a means to monitor the effectiveness of treatments aimed at slowing or preventing the formation of cataracts. "A medical trial of a cataract drug could take 10 years," notes Dr. Datiles. "With this device, we can detect loss or protection of alpha-crystallin in the lens to a high degree of accuracy. This could shorten a clinical trial of this kind to just a few years."
• Monitoring oxidative stress in unusal workplace conditions. "NASA is conducting a new study in which they're using the index we developed to look at oxidative stress in divers who train astronauts," says Dr. Datiles. "Astronauts spend a lot of time training underwater in a special pool. This exposes the divers who train them to high levels of oxygen, which may cause significant oxidative stress. NASA will soon be using our DLS device to monitor the amount of alpha-crystallin present before and after this experience, presuming that any decrease will reflect how much the other lens proteins have been damaged as a result of this stress. Dr. Ansari is also fixing our device inside a helmet that astronauts could put on briefly every day, allowing flight surgeons to monitor their protein levels safely and non-invasively."
What's Next?
Dr. Datiles says his next project is a longitudinal study of age-related cataract at the Wilmer Eye Institute in
"It's easy to recruit and follow patients at
The device itself should continue to evolve towards something that can be used on a daily basis in the clinic. "Right now, the DLS device is a small probe that you can attach to another device. We attached it to a 3-D aiming instrument so we can scan the same location again and again; the aiming part of the instrument isn't small, but should eventually become miniaturized. Hopefully, in the future this will evolve into a handheld probe, similar to a handheld tonometer. You'll be able to point it at a patient's lens, push a button and measure the alpha-crystallin. It's too early to know when something like this will be available, and the economy is currently shrinking research budgets. But when we get the right funding, we can go to the next step.
"Once a cataract appears, you'll still have to use another device to track the progression over time," he says. Unfortunately, by the time the cataract is visible, we don't know of any way to stop it. It becomes mainly an issue of deciding when it's time to do the surgery." For now, Dr. Datiles is anxious to spread the word about the function of alpha-crystallin in the human lens. "The concept of alpha-crystallin as a molecular chaperone is new for most clinicians, but it's extremely important in the biology of the lens and cataract formation," he says. "This technology should help bring that to the fore."
1. Datiles MB 3rd, Ansari RR, Suh KI, Vitale S, Reed GF, Zigler JS Jr, Ferris FL 3rd. Clinical detection of precataractous lens protein changes using dynamic light scattering. Arch Ophthalmol 2008;126:12:1687-93.