Whenever a major new approach to treating a condition arrives on the scene, it goes through a period of evolution during which the process is refined and variations are suggested and tried. Corneal collagen cross-linking, primarily used as a way to address keratoconus and corneal ectasia, is currently in the midst of that process.
The biggest evolutionary shift taking place today is the move from procedures that require removal of the epithelium to ones that don’t. However, other variations are being tried as well. Those include using cross-linking to cause refractive change; using cross-linking in combination with other procedures to increase the benefits experienced by the patient; miniaturizing the delivery system; triggering the cross-linking reaction chemically without UV light; and getting cross-linking to happen using oral riboflavin and sunlight.
Here, ophthalmologists and researchers share their work with each of these, provide updates on the current system approved by the FDA and discuss the status of other related ideas that are on the table.
Avedro/Glaukos: Epi-off & On
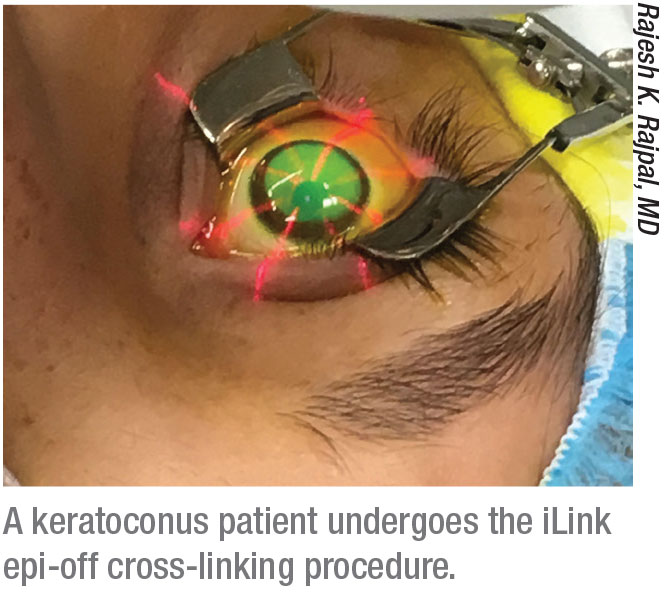
Currently, the only cross-linking system approved by the U.S. Food and Drug Administration is Glaukos’ iLink procedure (formerly known as Avedro’s corneal collagen cross-linking procedure. Avedro was recently purchased by Glaukos.) The iLink system includes two proprietary riboflavin formulations, Photrexa Viscous (riboflavin 5´-phosphate in 20% dextran ophthalmic solution) and Photrexa (riboflavin 5´-phosphate ophthalmic solution, a hypo-osmolar formula that can be used if a thin cornea needs to be swollen), plus a UV light source. “This system has been available since 2016, and it’s now well-accepted by cornea specialists and clinicians,” notes Rajesh K. Rajpal, MD, a clinical professor at George Washington University Medical Center and chief medical officer for Johnson & Johnson Vision (formerly CMO for Avedro).
“Our primary goal has always been to get patients better access to the procedure,” he says. “It took some time to accomplish, but the vast majority of insurers now cover it, and that’s been accompanied by a significant increase in its use. The transition from Avedro to Glaukos actually helped, because Glaukos has been able to provide even more supporting research, as well as providing financing options for purchasers and reimbursement support, which have made it easier for physicians to offer the cross-linking procedure and easier for patients to access it.”
Not surprisingly, the company is working hard to bring an epi-on system to market. “Leaving the epithelium on has obvious potential advantages in terms of safety, patient comfort and recovery time,” Dr. Rajpal says. “This could be especially beneficial for patients who need to minimize time away from school or work or whatever activities they may be doing.” He notes that the protocol under investigation also takes less time to perform. “It’s approximately a 30-minute treatment instead of a one-hour treatment,” he says. “The current iLink procedure requires 30 minutes of soak time and 30 minutes of light. The procedure under investigation is closer to 20 and 10 minutes, respectively, while the treatment goal remains the same.”
Dr. Rajpal says that the clinical Phase III studies of this epi-on cross-linking system have completed enrollment in the United States. “If the data meets the endpoint, the next step is submission to the FDA, with the potential for approval anticipated as early as 2022.”
Epi-on: Another Approach
 |
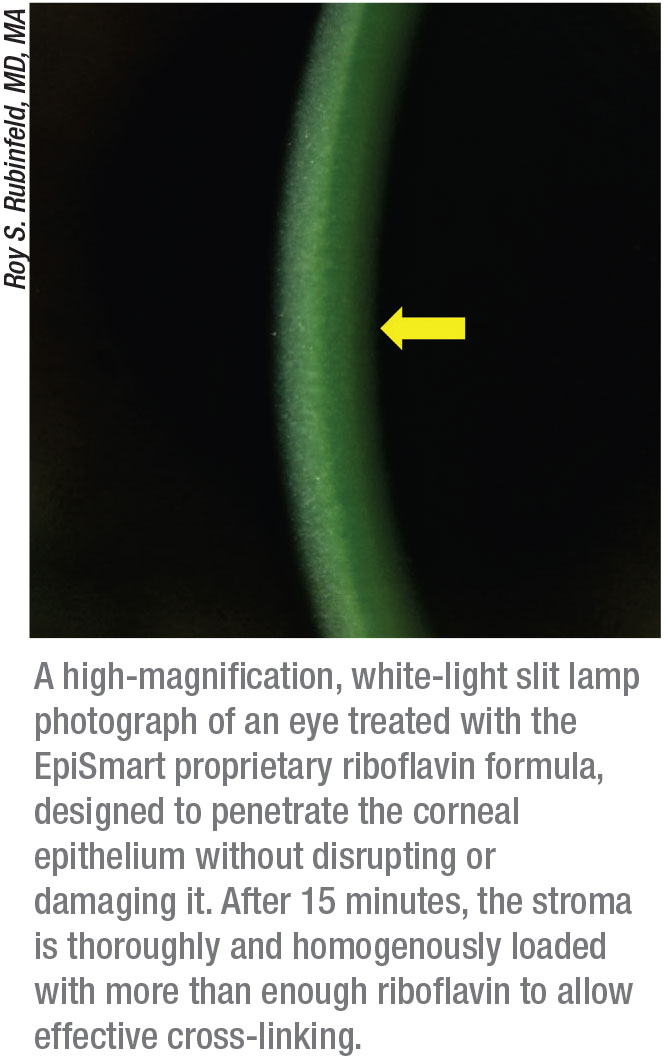
Roy S. Rubinfeld, MD, MA, a clinical associate professor at Georgetown University Medical Center and medical director at Re:Vision in Rockville, Maryland, and Fairfax, Virginia, has devoted extensive time to developing an effective epi-on cross-linking system. He says the current proprietary version of the epi-on system that he began developing years ago, now called EpiSmart (owned by CXL Ophthalmics, based in Encinitas, California), is preparing to enter the final stage of FDA clinical trials.
Dr. Rubinfeld describes how the EpiSmart system overcomes the traditional obstacles to epi-on cross-linking. “The first challenge is getting the riboflavin through the epithelium,” he explains. “Our proprietary riboflavin formula has been shown to rapidly, consistently and homogeneously penetrate intact epithelium and achieve corneal-stromal levels that allow excellent cross-linking.1 The second thing that’s different is our delivery system for the riboflavin. Many techniques have been tried that involve disrupting the epithelium via abrasion to make it more permeable, or driving the riboflavin through with the use of something like iontophoresis. Our system applies the riboflavin using patented non-abrasive, disposable, sponge-based delivery devices that are gentle on the corneal epithelium, easy-to-use and sterile.
“The third unique part of the system is the third-generation UV light delivery device,” he says. “It delivers the UV light in a patented pulse cycle that allows oxygen to rediffuse into the cornea during the procedure. Without adequate oxygen, the cross-linking reaction can create toxic reactive oxygen species like peroxides, with side effects like haze, cell death, scarring (which coincidentally causes corneal flattening) and corneal thinning. These innovations are additive and synergistic, and each one is critical.”
Dr. Rubinfeld points out one other significant advantage of the system. “Traditional cross-linking procedures are done one eye at a time,” he says. “That causes the patient a solid week of discomfort, and it takes many weeks for vision to return to preop levels. Then, after three months you start over and do the second eye. Our UV light source is designed for bilateral, simultaneous application, when indicated. By that evening or the following morning the patient’s vision is back to preop levels. The need to use opioids or bandage contact lenses is rare, as patients have just a few hours of typically mild to moderate discomfort.”
Evidence for Epi-on
Dr. Rubinfeld says that data published in the peer-reviewed literature has shown the EpiSmart system to be effective. “For example,” he says, “one publication describes 592 eyes treated with this system, 48 of which were pediatric eyes, which we know usually progress relatively quickly. In that paper, no eyes progressed after the treatment.2 Some have dismissed these data because we didn’t include a placebo cohort or require documented proof of progression to participate in the study. However, we believe that waiting for patients to lose vision before treating a known degenerative eye disease is archaic, much like waiting to treat glaucoma until some of the visual field has been lost. Furthermore, the risk-benefit ratio with epi-on is unique. The fact that none of the 592 eyes progressed speaks for itself.”
Parag A. Majmudar, MD, an associate professor of ophthalmology at Rush University Medical Center in Chicago and president and chief medical officer at Chicago Cornea Consultants, agrees. (He participated in the trials of the EpiSmart system for the past 10 years, but switched to the FDA-approved epi-off system when the trial he was participating in was completed.) He notes that requiring proof of progression in some clinical trials is having an unintended side effect. “We’ve seen leading insurance companies deny treatment for young patients who’ve started to lose vision simply because they don’t have that proof,” he says. “In addition, insurance companies give the flawed rationale that the patient sees well in contact lenses.”
Dr. Majmudar notes that having to switch to an epi-off procedure made the advantages of epi-on very clear. “Having only done the epi-off protocol for a year I can’t compare the long-term outcomes, but the number of patients having a much less pleasant experience and returning with corneal haze has been much greater. In the previous nine years I didn’t see a single case of postop haze in more than 2,500 epi-on procedures.”
Dr. Majmudar says that his personal experience with the epi-on system has been very positive. “Anecdotally, we’ve had a retreatment rate of about 1 to 2 percent, which is very comparable to the 2 to 3 percent within the first five years that’s been reported in the worldwide literature using the Dresden protocol,” he says. “We’ve had very high success rates and virtually no progression leading to the need for corneal transplantation during the entire nine years, which is, again, comparable to the Dresden protocol results. On top of that, we’ve had much better patient experiences.” Dr. Majmudar suspects that the challenging patient experience is one reason some surgeons haven’t been eager to offer epi-on cross-linking.
Dr. Rubinfeld says the EpiSmart system has now completed a Phase II trial and the company is moving toward Phase III.
CXL as a Refractive Treatment
Because the process of cross-linking tends to cause a small refractive change, the possibility of using an epi-on procedure for that express purpose has been under consideration for a number of years. A formalized version of that idea, known as photorefractive intrastromal cross-linking, or PIXL, is now undergoing testing by Glaukos. “Multicenter Phase II trials of PIXL are being conducted outside the United States,” notes Dr. Rajpal. “Those trials are designed to further refine treatment algorithms, evaluate outcomes and help the company determine the appropriate clinical and regulatory pathway in the United States. No specific timeline for that has been released.”
 |
Dr. Rajpal says the one target application anticipated for PIXL will be treating early presbyopia in patients who have fairly good distance vision. “This potential application makes sense in a patient with early presbyopia who is essentially emmetropic,” he explains. “In that situation, your goal is to make the nondominant eye myopic, giving the patient monovision.” (He notes that PRK and LASIK are rarely used to treat very low refractive errors because of the risk/reward balance.)
One of the issues that’s been raised with using a procedure such as PIXL to effect refractive change is the difficulty of achieving a precise level of change. “I don’t think this can give you the level of refractive precision you can get with PRK or LASIK, because it’s not removing tissue,” Dr. Rajpal acknowledges. “Instead, we’re changing the corneal shape, so it’s inevitably going to be less predictable. But I don’t think precision is the objective in this situation. When we’re treating presbyopia, even if the patient gets some degree of improvement—perhaps intermediate vision instead of near vision—there’s still a significant clinical benefit. In fact, in this situation patients are less critical about what the level of improvement is, as long as we’re improving their ability to see up close—to see their phones or tablets or computers. Patients understand that presbyopia is progressive, so eventually they’ll need another solution, but in the meantime, this could make a difference.
“Of course,” he adds, “all of this will depend on the findings in the clinical trials.”
Combination Procedures
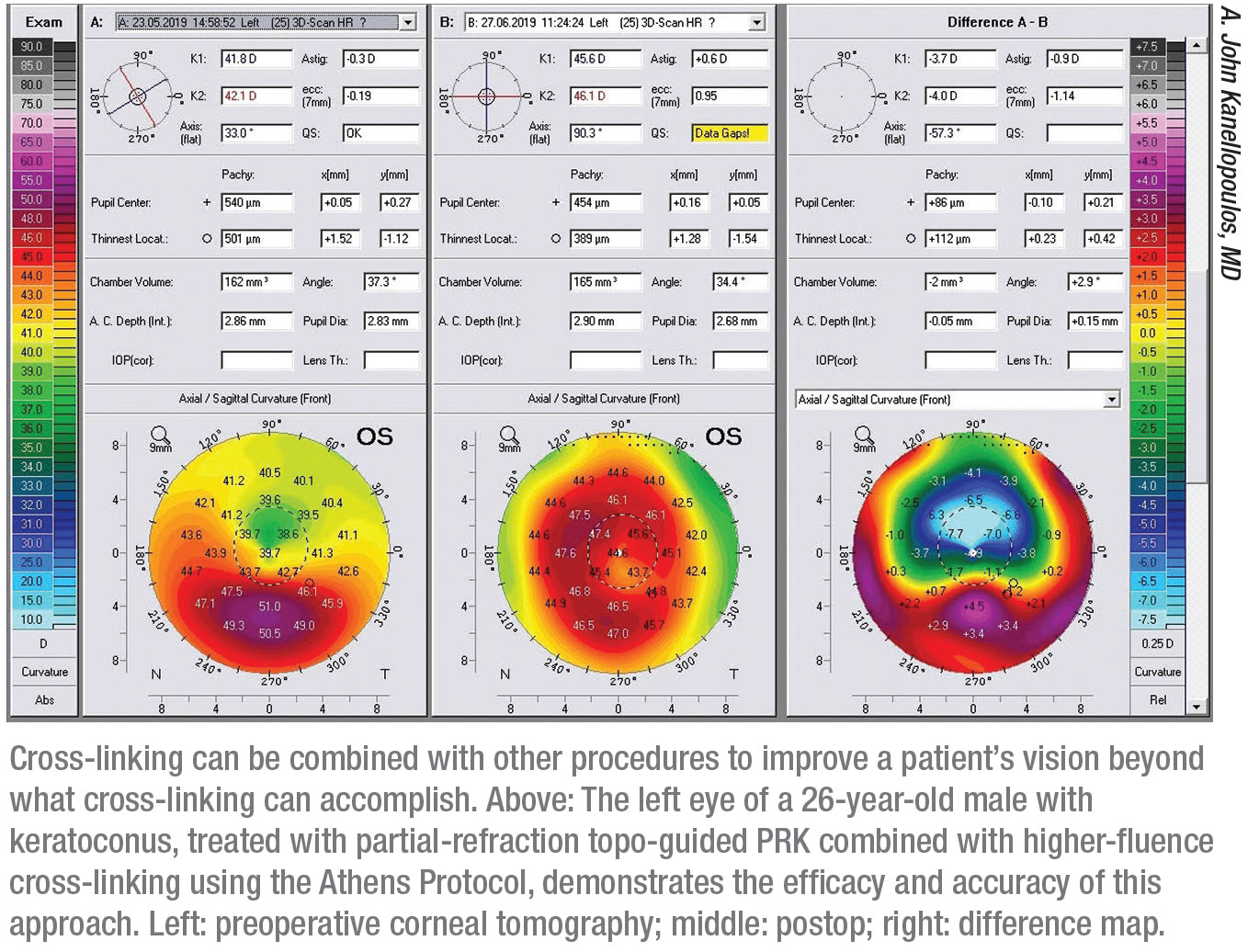
Dr. Rubinfeld believes combining cross-linking with other procedures such as PRK (off-label) may be very helpful in cases of keratoconus. “There’s been a lot of interest in combining treatments so that you not only stop progression but improve vision,” he says. “For example, doing some sort of topography-guided PRK that removes limited tissue, and then cross-linking a few weeks later to lock in the effect, is very promising. This could markedly improve the lives of patients whose disease was not caught early.”
“There are several techniques for combining cross-linking with a customized, partial-refraction ablation,” notes A. John Kanellopoulos, MD, a clinical professor of ophthalmology at NYU Medical School in New York City and medical director of the Laservision.gr Institute in Athens, Greece. “I’m proud to report that over the past few years my colleague Gregory Pamel, MD, and I have been able to combine topography-guided partial-refraction ablation with higher-fluence cross-linking in our New York City office, as well as in our Athens clinic. Our data, which mirror our European experience over the past 15 years, are currently under review for publication.”
If procedures are combined, there’s some debate about the order and timing of the procedures. “There have been cases of patients having cross-linking and PRK on the same day and ending up with a refractive surprise,” Dr. Majmudar points out. “If you do the procedures sequentially with some time in between, you might be able to better predict the outcome. Of course, this would be less of an issue if your goal is not to free the patient from wearing glasses, but simply to eliminate some of the higher-order corneal aberrations using topography-guided treatment.”
Dr. Rajpal says he believes that if procedures are done in combination, it’s logical to do the cross-linking first. “That way, you’ll know how much change has been induced by the cross-linking,” he notes. “If you do them at the same time and then the cornea changes because of the cross-linking, your refractive outcome may not be as predictable. Of course, this will come down to physician discretion.”
An On-eye System
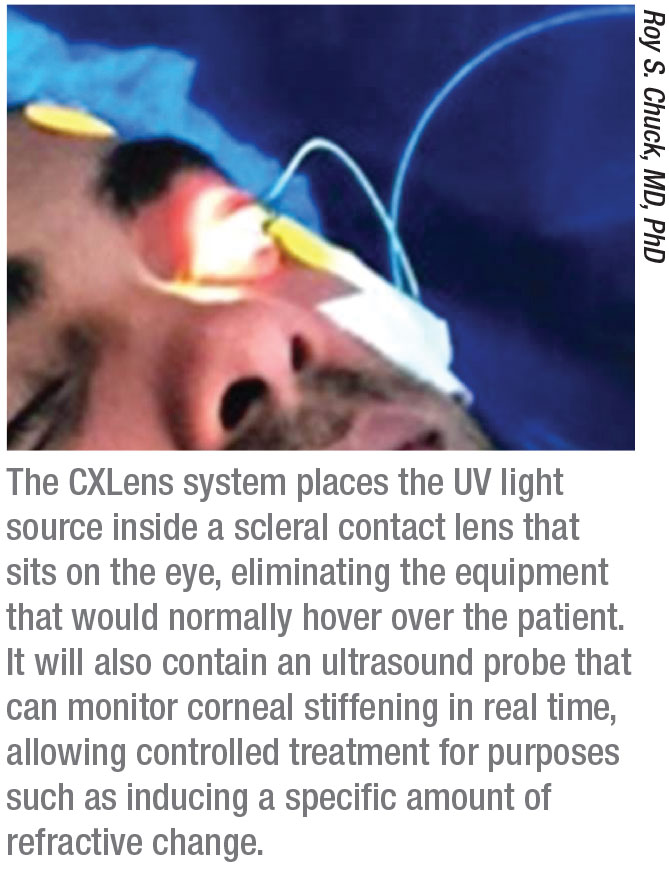
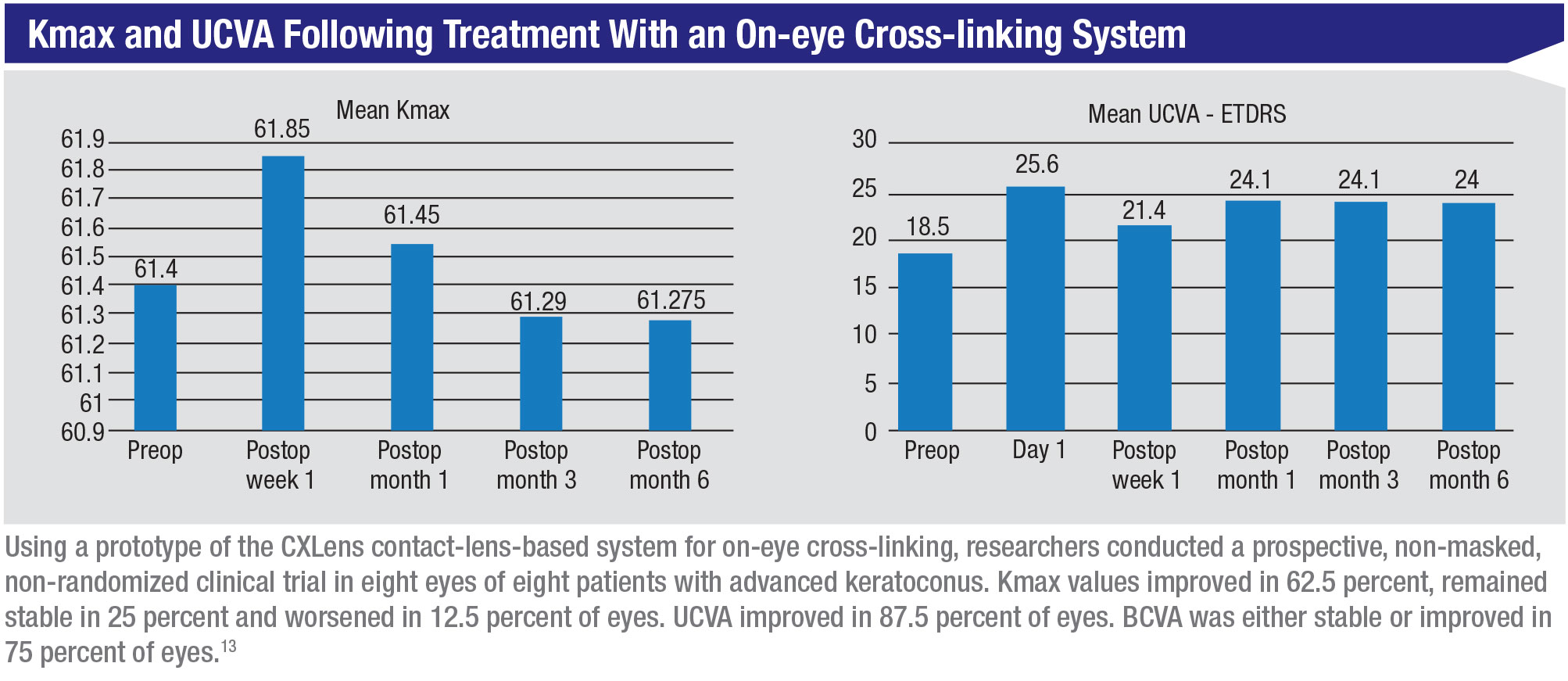
One revolutionary new approach to performing cross-linking has been developed by Roy S. Chuck, MD, PhD, the Paul Henkind Chairman in the Department of Ophthalmology and Visual Sciences at Albert Einstein College of Medicine, Montefiore Medical Center in New York City, working with David Acker, a medical device entrepreneur, and Patrick Lopath, a biomedical engineer. They’ve found a way to miniaturize the UV light delivery system so that it can be placed directly on the eye, a system they’re calling the CXLens. This miniaturization, in turn, makes it possible to monitor changes in the cornea in real time during the procedure.
“Several years ago I became interested in the idea of finding a way to make the cross-linking process easier for everyone by turning all of the cross-linking equipment into a wearable technology,” Dr. Chuck explains. “Instead of the UV light source being in an overhead lamp, our team put it inside a scleral contact lens—designed with help from colleague Deborah S. Jacobs, MD—which rides above the cornea, resting on the sclera. (See picture, p. 40.) This allows the patient to be ambulatory during the procedure.
 |
“One of the benefits of this approach is that there’s no need to use an eye tracking system to ensure correct irradiation,” he continues. “Some cross-linking systems use an eye tracker that only notes when the eye turns away, shutting off the light in response. With our system, if the eye moves, the contact lens moves with it. Also, there’s no speculum holding the eye open; in fact, the patient can close his or her eyes over the device. As a result, it’s more comfortable.”
Dr. Chuck and his colleagues realized they could add another key piece of technology to make this system even more useful. “We’ve embedded a tiny ultrasound probe into the apex of the lens,” Dr. Chuck explains. “This allows us to track the amount of corneal stiffening taking place in real time. This could be useful if you’re treating keratoconus, but it’s critical if you’re using corneal cross-linking to alter the patient’s refraction. When you can track the amount of corneal stiffening, you can directly correlate that with refractive change and modulate the treatment accordingly.” (Dr. Chuck was an early proponent of the possibility of using cross-linking to cause a refractive change.3)
“Knowing exactly how much change you’re causing has been an issue with every refractive treatment,” he notes. “Even with PRK and LASIK, in the early days no one knew how much energy to use to get the desired refractive change. It wasn’t until we had a lot of population data that nomograms were developed. Real-time monitoring is even more important when using cross-linking to generate refractive change, since this involves tissue remodeling rather than tissue ablation.”
As with every cross-linking system, two key issues tied to effectiveness are getting the riboflavin into the tissue and making sure sufficient oxygen is present for the reaction to occur in the desired way. Given the CXLens’ radically different treatment setup, Dr. Chuck explains how they’ve addressed these two issues.
“The way surgeons get the riboflavin into the tissue in a standard procedure has several drawbacks, such as the need for a nurse to put drops in the patient’s eye every few minutes,” he notes. “So, the first thing we did was to take a scleral lens—separate from the treatment lens—and use it as a reservoir. We put our riboflavin solution into the lens, place it onto the eye and then leave. When the tissue is sufficiently saturated, we switch to the treatment lens.
 |
“Second, we’ve been developing our own formulation of riboflavin that can get past the epithelium without damaging it,” he continues. “For the pilot trials we had to use a currently available off-the-shelf trans-epithelial solution. These formulations aren’t ideal, as they use preservatives to disrupt the epithelium enough to allow passage of the riboflavin. The riboflavin formulation we’re developing opens up the channels between the epithelial cells without damaging them. It’s a technology invented by John Tomich, PhD, a biochemistry professor at Kansas State University; renowned ophthalmic researcher Henry Edelhauser, PhD, also spent time working on it near the end of his life. We acquired it from Kansas State University and are working with Dr. Tomich to perfect it. Once applied, our solution keeps those channels open for 90 minutes, letting the riboflavin pass through the epithelium. After that, the epithelium returns to its normal state.”
The other hurdle to overcome is making sure the cornea has plenty of oxygen during the procedure. “The contact lens does allow oxygen to pass through, but that’s not sufficient,” says Dr. Chuck. “We’ve developed a patent-protected method to use a special fluid as a very efficient carrier of oxygen; divers and intensive-care units in hospitals sometimes use a variation on this to help compensate for oxygen deprivation. We fill the space between the lens and the cornea with this fluid and it keeps the cornea oxygenated without the need to blow air across it.”
Dr. Chuck says they were able to complete a proof-of-concept trial involving 10 patients with advanced keratoconus in late 2019. “Fortunately, we were able to complete the three- and six-month follow-ups before COVID became an issue,” he says. “While we didn’t use our riboflavin formulation in this trial, the clinical results were very good. Now we’re in discussions with the FDA about taking this to the next stage, which will involve treating keratoconus and doing refractive cross-linking trials.”
Cross-linking Via an Eye Drop
One of the latest potential approaches to addressing keratoconus bypasses the use of riboflavin and UV light altogether. A twice-daily eye drop called IVMED-80, being developed by iVeena Delivery Systems in Salt Lake City, appears to be able to stop the progression of keratoconus (according to current data going out six months). The idea for this approach came from Bala Ambati, MD, president of the Pacific Clear Vision Institute in Eugene, Oregon, and a research professor at the University of Oregon. (Dr. Ambati is also the president and co-founder of iVeena.)
“As a cornea specialist I’m well aware of the challenges of epi-off corneal cross-linking,” Dr. Ambati explains. “In the course of my research as a clinician scientist I noted that lysyl oxidase, a natural enzyme in the cornea, mediates cross-linking. In fact, a deficiency of lysyl oxidase has been associated with keratoconus in multiple studies, which makes sense given the inadequate existing cross-linking found in these patients.4-9 One particularly nice study from Rohit Shetty, MD, examined the lenticules from patients who underwent the SMILE procedure and later developed keratoconus; the lenticules from those patients had below-normal levels of lysyl oxidase.10
“That got me thinking: If lysyl oxidase deficiency is a problem, how do we restore it to normal levels?” he continues. “Further research revealed that copper is a key element in lysyl oxidase activity, and that it, too, is reduced in keratoconic corneas. So, when we started iVeena in 2015, the first question we asked was: How can we increase lysyl oxidase to promote physiologic cross-linking in corneal tissue? The first obvious avenue to try was an eye drop containing copper. In one of our early experiments we tried a proprietary solution on corneal cells taken from healthy human corneas, or from keratoconus patients who’d undergone a corneal transplant. The solution did indeed increase lysyl oxidase activity in the cells.
“Next, we tested the IVMED-80 solution in rabbits and found that it increased cross-linking in the living rabbit eye,” he says. “Then we tried using the eye drops in the rabbit eyes twice a day for about seven weeks, and the corneas exhibited about 1.7 D
of flattening. Most importantly, the drops were well-tolerated. There was no inflammation, no redness, no opacity and no damage to any of the rabbit ocular tissues. We also looked for copper accumulation in the plasma or liver or kidneys and found none.”
Dr. Ambati says they next moved on to a Phase I/IIa clinical study in patients. “We started the study in March, 2019 in Mexico, enrolling a series of 33 patients with keratoconus,” he says. “The study concluded this summer. One-third of the patients received placebo; one-third received the IVMED-80 drop for about six weeks, and another third received the drop for 16 weeks. We followed everybody for six months. By structuring the study this way we were able to evaluate the impact of the duration of therapy, as well as what would happen after stopping therapy.
“The overall results were excellent,” he continues. “At six months the patients who received the drug for 16 weeks had a K-max that was 2.3 D flatter than placebo. The patients who received the drug for six weeks got a little bit better but then returned to baseline by the end of the six months. Most importantly, there were no treatment-related adverse events. There was no inflammation, pain or redness induced by the drug. It was very well tolerated, with no issues.
“Based on the averages, it looks like there was no regression in the 16-week group after stopping the drug,” he adds. “We’ll see if that was true for every patient when we analyze each patient individually. I think duration of treatment may end up depending on age and severity of progression.”
Dr. Ambati says his team will be completing the statistical analysis of the trial data over the next couple of months. “We should have a final report before the end of the year,” he says. “The next step is to proceed with the U.S. clinical development pathway, and we’re already working with the FDA. Hopefully we can start the Phase III studies late next year.”
 |
Treating With Oral Riboflavin
In the past few years, John S. Jarstad, MD, FAAO, a professor of clinical ophthalmology at the Morsani School of Medicine, University of South Florida-Tampa, has been working with a very low-tech, inexpensive approach to cross-linking he stumbled onto a few years ago that’s showing promise in clinical testing. In this protocol, patients simply take an oral riboflavin supplement every day and walk vigorously (to increase oxygenation) toward the sun at midday for 15 minutes each day without wearing sunglasses or UV-blocking contact lenses. Remarkably, he says this protocol seems to produce results equal to those seen with the more high-tech, costly cross-linking treatments. The trade-off is that it takes several months to be effective.
Dr. Jarstad came up with the idea when trying to help a desperate patient who couldn’t afford the high-tech options, which weren’t covered by her insurance. In the clinical trial he conducted to test this approach (NCT03095235 at the NIH trial website) every patient taking 400 mg/day of riboflavin and following the walking protocol—except for one patient who never removed his UV-blocking contact lenses—achieved corneal flattening (average: 1.2 D), with keratoconus progression halted. A control group taking 100 mg/day also stabilized but saw less corneal flattening. Effects have been observed within three months, but the best results have generally been measured at six months.
A commonly raised concern is possible toxicity from the oral riboflavin. Dr. Jarstad reports that no adverse effects have been observed in any patients, noting that one colleague reported that a patient took 1,500 mg per day with no side effects (and an even greater flattening). He also notes that he hasn’t found any reports of complications associated with high-dose riboflavin in the literature.
Recently, Dr. Jarstad has noted that this approach also seems to be helping patients who have previously undergone radial keratotomy. “One patient in our study had eight-incision RK in 2008 and suffered from fluctuating vision for many years,” he says. “When I first saw her in May of 2016, as she began our study, she had post-RK ectasia with K readings of 33.9 by 35.6 D in her right eye and 35.9 by 37 D in her left eye. Her best-corrected vision measured 20/40 in the right eye and 20/30 in the left eye. After participating in the protocol her keratometry readings stabilized, and they’ve been stable since then. In June of this year, her visual acuity measured 20/20 without correction and her keratometry readings showed less than 0.75 D of astigmatism in each eye.”
The low cost and positive results are generating interest in other countries. Leonardo Torquetti, MD, PhD, an ophthalmologist and cornea specialist from Brazil who treats many keratoconus patients, has been trying this protocol on keratoconus patients who are at risk of progression. He reports that none of those patients have progressed since he began this treatment. He reports that other doctors in Brazil are trying the protocol as well.
Another remarkable finding reported by Dr. Jarstad has been that his study of this protocol also appears to confirm reports in the literature that oral riboflavin can provide major relief from migraine headaches and ophthalmic migraine. “We first noticed this when patients in our study with a history of migraines reported that their migraines had stopped,” he says. “Following this discovery we began using this approach to treat patients with ophthalmic and classic migraine symptoms, including patients with intractable migraine. So far, in 79 out of 80 patients treated with 100 mg of riboflavin capsules taken by mouth per day—increasing by 100 mg each week up to 400 mg/day for those not responsive to 100 mg/day—we’ve seen a cure of their migraine variants.”
 |
Other Options and Issues
As corneal cross-linking continues to evolve, different methods for accomplishing it continue to appear, while some past protocols are falling by the wayside.
• Altering parameters to speed the process. “The top cross-linking photochemists that I’ve talked to consider this idea to be ill-advised,” says Dr. Rubinfeld. “This premise is based on the Bunsen-Roscoe law of reciprocity, which suggests that the results of 5 minutes of 50 mW/cm2 UVA exposure has the same effect as 50 minutes of
5 mW/cm2. This law applies in the photography darkroom, but not in the biological world. Also, the rate-limiting factor is oxygen, not UV light. The majority of the peer-reviewed literature suggests that accelerated cross-linking is inferior to standard treatment. You may cut the length of the procedure by a few minutes, but the outcomes generally aren’t as good.”
• Loading the riboflavin into a stromal pocket. Getting the riboflavin into the stromal tissue via a femtosecond-laser-created cornea pocket (similar to that created for the SMILE procedure) is an idea that was pioneered by Dr. Kanellopoulos’ group about 10 years ago, as a way to avoid having to remove the epithelium. However, the positive results being seen with other less-invasive epi-on approaches has prevented this idea from becoming widely used. “The stromal pocket approach is currently mostly an investigative tool,” Dr. Kanellopoulos says.
• Approaches that compensate for thin corneas. The idea that UV light might damage the endothelium if the cornea is too thin—i.e., less than 400 µm thick—was proposed by Gregor Wollensak, MD, in 2004.11 This has led to several protocols that can be used to ensure that corneas are at least that thick prior to cross-linking. However, the validity of that requirement is now being challenged.
“Subsequent studies of the epithelium have demonstrated that 400 µm is not a magic number below which cross-linking will be a disaster,” says Dr. Rubinfeld. “In fact, Mooren et al conducted a study in which they took a human cornea, put some riboflavin in and turned it upside down; then they directly irradiated the endothelium with continuous UVA of 18 mW/cm2.12 While acknowledging the limitations of an ex vivo study, the authors conclude from their findings that human corneal endothelial cells are more resistant to UVA irradiation in the presence of riboflavin than was previously estimated based on animal experiments. This suggests that many of the protocols that have been developed to ensure that cross-linked corneas are at least 400 µm thick may be unnecessary, if the corneas are treated with cross-linking systems that balance the cross-linking reaction.
“In fact,” he adds, “our epi-on technology has safely treated a large number of very thin corneas without swelling them or using a contact lens.”
• Customizing the procedure based on corneal thickness. Dr.
Rajpal believes that customizing treatment based on individual corneal characteristics is a worthwhile goal. “Ultimately I think we’ll find ways to do that as we gather more data and understand patient outcomes better,” he says. “The idea of doing less treatment or modifying where the treatment is done based on the thickness of the cornea or steepness of the cornea may be achievable once there are adequate clinical data available.”
Dr. Kanellopoulos notes that his group has done a lot of work with customized cross-linking since 2013, using the Avedro Mosaic device. “It makes sense as a way to employ cross-linking to cause refractive changes in the cornea in a more controlled and predictable fashion,” he says. “I think it holds great promise for the future.”
Dr. Rubinfeld, however, is skeptical. “This is an interesting concept, but to what end?” he asks. “The primary use for this procedure is to improve or maintain vision and prevent further deterioration in cases of keratoconus and ectasia. I don’t think this type of customization would make much difference.”
• Using iontophoresis to drive the riboflavin through the epithelium. Dr. Rubinfeld notes that this approach isn’t widely used. “It’s extremely painful,” he says. “We started using it in 2010 or 2011 but stopped using it by 2012. In recent years, when I lectured in Europe I’d ask if anyone had used iontophoresis and most hands would go up. Then I’d ask if anyone was still using it, and pretty much everybody dropped their hands.”
Dr. Kanellopoulos agrees that there’s little evidence of its current use. “It seems that its initial advantage in epi-on cross-linking has been bypassed by other topical agents that can achieve the same result,” he says.
Good Medicine in Any Form
No matter which cross-linking approach (or approaches) prevail, Dr. Kanellopoulos believes that cross-linking will have a major impact on how corneal problems such as ectasia and keratoconus are addressed. “If clinicians around the world become astute in diagnosing these conditions and offering treatment early,” he says, “we should be able to eliminate the need for cornea transplantation.” REVIEW
Dr. Rajpal is CMO at Johnson & Johnson Vision and a consultant for Glaukos. Dr. Rubinfeld is on the board of CXLO, is a named inventor on all of the U.S. and European patents for its cross-linking system and is managing member of CurveRight LLC. Dr. Majmudar is a consultant for Alcon and an investor in the CXLO EpiSmart epi-on system. Dr. Kanellopoulos is a consultant for Avedro and Alcon. Dr. Chuck is co-founder and co-owner of TECLens, developer of the CXLens. Dr. Ambati is president and co-founder of iVeena and has an equity interest in the company. Dr. Jarstad reports no relevant financial ties.
1. Rubinfeld RS, Stulting RD, Gum GG, Talamo JH. Quantitative analysis of corneal stromal riboflavin concentration without epithelial removal. J Cataract Refract Surg 2018;44:2:237-242.
2. Stulting RD, Trattler WB, Woolfson JM, Rubinfeld RS. Corneal cross-linking without epithelial removal. J Cataract Refract Surg 2018;44:11:1363-1370.
3. Park S, Chuck RS. Corneal collagen cross-linking for correction of low myopia? Curr Opin Ophthalmol 2013;24:4:273-4.
4. Bykhovskaya Y, Li X, Epifantseva I, et al. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest Ophthalmol Vis Sci 2012;53:7:4152-7.
5. Dudakova L, Jirsova K. The impairment of lysyl oxidase in keratoconus and in keratoconus-associated disorders. J Neural Transm (Vienna) 2013;120:6:977-82.
6. Hasanian-Langroudi F, Saravani R, et al. Association of lysyl oxidase (LOX) polymorphisms with the risk of keratoconus in an Iranian population. Ophthalmic Genet 2015;36:4:309-14.
7. Dudakova L, Liskova P, et al. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp Eye Res 2012;104:74-81.
8. Dudakova L, Palos M, et al. Validation of rs2956540:G>C and rs3735520:G>A association with keratoconus in a population of European descent. Eur J Hum Genet. 2015;23:11:1581-3.
9. Zhang J, Zhang L, et al. Association of common variants in LOX with keratoconus: A meta-analysis. PLoS One 2015;10:12:e0145815.
10. Shetty R, Kumar NR, Khamar P, et al. Bilaterally asymmetric corneal ectasia following SMILE with asymmetrically reduced stromal molecular markers. J Refract Surg 2019;35:1:6-14.
11. Wollensak G, Spoerl E, Wilsch M, et al. Keratocyte apoptosis after corneal collagen cross-linking using riboflavin/UVA treatment. Cornea 2004;23:43–49.
12. Mooren P, Gobin L, Bostan N, et al. Evaluation of UVA cytotoxicity for human endothelium in an ex vivo corneal cross-linking experimental setting. J Refract Surg 2016;32:41–46.
13. Logrono JB, Duran CR. Trans-epithelial (epithelium-on) corneal collagen crosslinking using a novel ultraviolet light-emitting contact lens device. Presented at the 2020 annual meeting of the American Society of Cataract and Refractive Surgery.




