Anti-VEGF drugs for wet AMD are not all created equal. Available anti-VEGF agents, such as pegaptanib sodium (Macugen), bevacizumab (Avastin) and ranibizumab (Lucentis) come from two established drug classes. Cutting edge investigational drugs such as VEGF-Trap and Cand5 represent new classes of agents just now undergoing clinical trials in ophthalmology. The monoclonal antibody ranibizumab was the first anti-VEGF agent to improve visual acuity in wet AMD patients, and upcoming anti-VEGF therapies may improve on Lucentis' success by inhibiting VEGF-A isoforms in unique and potentially more effective ways. Here's a look at the mechanisms behind the new treatments.
Fusion Proteins
The genetic recombination technique was first described in 1973 and experimentally demonstrated in 1978 by Nobel prize-winning researchers Werner Arber, PhD, Hamilton Smith, MD, and Daniel Nathans, MD.1 Recombinant proteins are secreted from genetically engineered cells with foreign DNA (rDNA) inserted, or recombined, into their genetic code. The first rProtein therapy, tissue plasminogen activator (tPA) was approved by the U.S. Food and Drug Administration in 1987 to dissolve blood clots. Another notable rProtein, erythropoietin, or EPO (Epogen, Amgen), was approved to treat anemia in 1989.
The latest leap in the evolution of recombinant technology is fusion protein synthesis. To make a gene that encodes a fusion protein, one protein's DNA—with the stop codon deleted—is attached, or "fused," with the DNA of another protein. The resultant artificial gene is then spliced into a cell that has been selected to manufacture the fusion protein. The most widely used anti-tumor necrosis factor (TNF) biologic in rheumatology, etanercept (Enbrel, Immunex), is a fusion protein. Enbrel, which is FDA-approved for rheumatoid arthritis and other inflammatory conditions, is formed from a portion of the TNF cytokine and the constant region of the human immunoglobulin-1 (IgG1) protein. We have yet to benefit from this technology, but the first fusion protein drug for wet AMD is in development.
VEGF-Trap is the first anti-VEGF agent to use genetically engineered decoy receptors to inhibit wet AMD. VEGF-Trap is made from the DNA portions of the principal binding domains of VEGF receptors 1 and 2 (VEGFR1 and VEGFR2) and the gene sequence for human IgG1.2 The resultant fusion protein tightly binds to all isoforms of VEGF-A and both forms of Placental Growth Factor (PlGF1 and 2). VEGF Trap's efficient stoichiometric relationship and high binding affinity means it is effective at tissue concentrations that are considerably lower than other anti-VEGF agents, such as ranibizumab, bevacizumab and pegaptanib sodium. The IgG1 portion of the molecule gives VEGF-Trap a long circulating half-life and may also increase its residence time in the eye. Regeneron Pharmaceuticals is currently conducting a Phase II study of the VEGF-Trap in patients with active CNV due to AMD.
RNAi
Another emerging technique that could yield a successful anti-VEGF therapy is RNA interference (RNAi). In 1998, researchers noticed that when short, double-stranded RNA was injected intracellularly, it hybridized readily with the endogenous RNA.3 The possibility of artificial manipulation of gene expression encouraged the development of many small interfering RNA (siRNA) therapies. In 2002, siRNAs were used to accurately target the CD4 HIV-1 receptor in vitro.4 Acuity Pharmaceuticals is currently conducting a three-dose safety study of Cand5, an anti-VEGF siRNA for wet AMD.
SiRNAs are 21-23 base pair (bp) fragments of double-stranded RNA (dsRNA) molecules broken by an RNAse III nuclease. When integrated into the cleavage catalyst RNA-induced silencing complex (RISC), siRNAs target and destroy specific native mRNAs. A RISC guided by the right siRNA could preemptively block VEGF by destroying the mRNA that manufactures it (See Figure 1). SiRNAs are delivered inside the capsid of an adenovirus5 or retrovirus,6 and may activate the nonspecific antiviral immune response before implantation. Although extracellularly siRNAs are associated with side effects, intracellularly siRNAs have a precise specificity and don't affect DNA. There is currently no clinical data on Cand5, the first siRNA tested in ophthalmology.
Approved Anti-VEGFs
Monoclonal antibodies (MAbs) were first isolated in 19757 by Georges Kohler, PhD, Niels Jerne, MD, PhD, and Cesar Milstein, PhD, who were awarded the 1984 Nobel Prize in Medicine for their work. To create monoclonal antibodies, spleen cells are harvested from a lab mouse induced to produce an antibody, and fused with a myeloma tumor cell.8 The resultant cell, called a hybridoma, grows indefinitely at the rate of a cancer cell, while producing the same antibodies as the original mouse B-lymphocyte. Because every clone of the hybridoma produces the exact same antibody, MAbs are specific for particular antigens. Ranibizumab (Lucentis), the newest anti-VEGF monoclonal antibody, is thought to block all VEGF-A isoforms. Bevacizumab (Avastin), a colorectal cancer drug used off-label to treat wet AMD, is also a monoclonal antibody and a VEGF-A antagonist.
Chemically, Macugen, the first FDA-approved anti-VEGF, is a synthesized short-stranded RNA (oligonucleotide) called an aptamer.9 Macugen was created in vitro using the SELEX (Systematic Evolution of Ligands of Exponential enrichment) combinatorial chemistry process to bind to extracellular VEGF.10 Macugen selectively binds to one VEGF-A isoform, VEGF165, an inflammatory and angiogenic factor.11
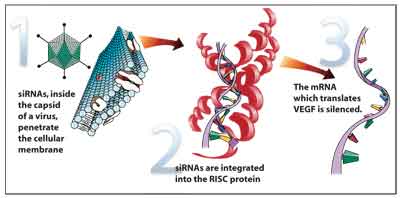
Figure 1. A RISC protein is guided by a siRNA to block VEGF.
While both monoclonal antibodies and aptamers can be specific for VEGF, the method of their manufacture is essentially a refined version of trial and error. Recombinant fusion proteins such as VEGF-Trap and small interfering RNAs like Cand5 are developed in a more controlled and specific manner to optimize effectiveness. Although clinical data on these investigational drugs is not complete, the method of their fabrication shows great promise.
Administration
There remain many wet AMD patients who don't respond to currently available monoclonal antibodies and aptamers such as Lucentis, Avastin and Macugen. The unique molecular composition of VEGF-Trap may address the needs of some of these patients. VEGF-Trap remains present in the posterior segment for more than a month, and effectively binds to VEGF at lower ambient concentrations than traditional anti-VEGF agents.2 Preliminary data indicates that VEGF-Trap may require less frequent injections than other anti-VEGFs such as Lucentis. Since the complications associated with intravitreal injection occur in proportion to the number of retreatments, VEGF-Trap's long duration of action may translate into a lower incidence of delivery-related side effects and increased comfort.
Prolonged AMD Treatment
Medications such as Lucentis have proven VEGF antagonists can dramatically benefit wet AMD patients in the short term. In the long term, however, the appropriate treatment regimen has not been determined. For chronic AMD, safety is a significant concern. The long-term effect of systemic VEGF down-regulation is unknown, but might interfere with VEGF's positive physiologic role as a survival factor for neurons,12 endothelial cells and the retinal pigment epithelium. Additionally, the prospect of lifelong intravitreal injections may be discouraging to some patients. A multi-step approach, such as using an anti-VEGF treatment for acute AMD and a safer angiostatic therapy to treat chronic AMD, should be considered.
For chronic management of wet AMD, a drug such as anecortave acetate (Retaane, Alcon) could be a good complement to a combinationtherapy regimen. The drug is delivered via a non-invasive posterior juxtascleral depot in contact with the posterior scleral surface and has not been associated with any serious complications after thousands of injections worldwide.13 Further, Retaane has the potential to attenuate the angiogenic cascade both upstream and downstream of traditional anti-VEGF compounds, and only needs to be administered every six months. SiRNAs such as Cand5, which disrupt the neovascular process upstream of anti-VEGFs, should be considered for
chronic treatment as well.
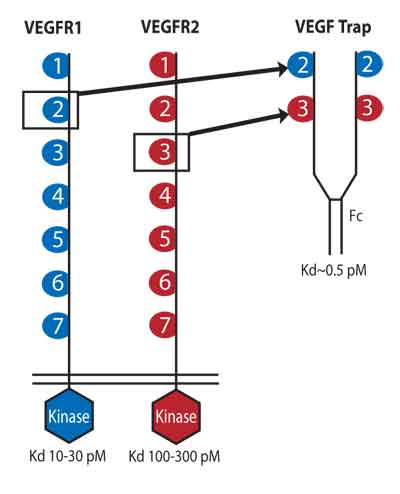
VEGF-Trap is derived from a VEGF receptor, and components from human immunoglobulin.
The Future of AMD
AMD patients today are much more likely to retain their central vision than patients diagnosed even a few years ago. A number of presently available drugs can maintain and even improve visual acuity in wet AMD, and there is reason to be optimistic about future management of the disease as well. The steady stream of epidemiological and etiological research being done on this degenerative condition will continue to aid the development of new treatments.
In the near future, ophthalmologists will have the opportunity to use some of the first therapies based on some of the newest breakthroughs in genomics. VEGF-Trap, a fusion protein conceptually similar to the anti-TNF agent Enbrel, is currently on the verge of Phase III clinical trials, and Cand5 will be the first siRNA therapy in the history of medicine. Both of these drug classes have the potential to significantly improve the prognoses of many wet AMD patients. Combination therapies, such as an anti-VEGF with Retaane or a siRNA, have the potential to safely and effectively improve the visual acuity of patients with chronic wet AMD. In short, the pipeline of drugs in development is not only full, it's full of promise.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals. Dr. Hartnett, an associate professor of ophthalmology at the University of North Carolina School of Medicine, is a vitreoretinal surgeon and the principal investigator at an NIH-supported laboratory at University of North Carolina, Chapel Hill. Mr. McLaughlin is a medical writer at ORA Clinical Research and Development in North Andover.
1. Raju TN. The Nobel chronicles, 1978. Lancet 1999;354:9189:1567.
2. Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. PNAS 2002;99:12:11393-11398.
3. Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391:6669:806-11.
4. Novina CD, Murray MF, Dykxhoorn DM, et al. siRNA-directed inhibition of HIV-1 infection. Nat Med 2002;8:7:681-6.
5. Shen C, Andreas KB, Liu X, et al. Gene silencing by adenovirus siRNA. Federation of European Biochemical Societies Letters 2003;539:111-114.
6. Devroe E, Silver PA. Retrovirus-delivered siRNA. BMC Biotechnology 2002;2:15.
7. Köhler, G Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975;256:495-497.
8. Ward PA, Adams J, Faustman D, et al. For the Committee on Methods of Producing Monoclonal Antibodies. Monoclonal antibody production. National Academy Press 1999.
9. Bunka DH, Stockley PG. Aptamers come of age— at last. Nat Rev Microbiol 2006;4:8:588-96.
10. Drolet DW, Jenison RD, Smith DE, et al. A high throughput platform for systemic evolution of ligands by exponential enrichment (SELEX). Comb Chem High Throughput Screen 1999;2:5:271-8.
11. Usui T, Ishida S, Yamashiro K et al. VEGF164(165) as the pathological isoform: differential leukocyte and endothelial responses through VEGFR1 and VEGFR2. Invest Ophthalmol Vis Sci 2004;45:368-74.
12. Liu MH, Jin H, Floten HS, et al. Vascular endothelial growth factor-mediated, endothelium-dependant relaxation in human internal mammary artery. Ann Thorac Surg 2002;73:819-824.
13. Augustin AJ, D'Amico DJ, Mieler WF, Schneebaum C, Beasley C. Safety of posterior juxtascleral depot administration of the angiostatic cortisene anecortave acetate for treatment of subfoveal choroidal neovascularization in patients with age-related macular degeneration. Graefe's Arch Clin Exp Ophthalmol 2005;243:1:9-12.



