A number of exciting, cutting-edge retinal treatments are being investigated against the backdrop of Spark’s Luxturna being approved by the Food and Drug Administration last year. Here’s a sampling of some promising new drugs and gene therapies.
Next-Generation Anti-VEGF
According to Pravin Dugel, MD, who is in practice in Phoenix, one of the most exciting drugs in this category is brolucizumab (Novartis), which is a single-strand antibody fragment. “The Phase III trials, HAWK and HARRIER, have shown anatomic superiority over aflibercept. This is the next drug in the pipeline that will change our management,” he says.
The HAWK study included neovascular AMD patients randomized 1:1:1 into three treatment groups: 358 patients received 3 mg of brolucizumab, 360 patients received 6 mg of brolucizumab, and 360 patients received 2 mg of aflibercept (Eylea, Regeneron).1
The HARRIER study included neovascular AMD patients randomized 1:1 into two treatment groups: 370 patients received 6 mg of brolucizumab and 369 patients received 2 mg of aflibercept.1
In both studies, patients in the brolucizumab group received three loading doses and then were treated every 12 weeks during the maintenance phase. Patients in the aflibercept group were treated every eight weeks.
In HAWK and HARRIER, the mean change in best-corrected visual acuity from baseline to week 48 was noninferior for brolucizumab compared with aflibercept. In the HAWK study, brolucizumab patients who received 3 mg experienced a mean BCVA gain of 6.1 letters, and those who received 6 mg improved by 6.6 letters, compared with 6.8 letters for the patients who received 2 mg of aflibercept.
In the HARRIER study, patients who received 6 mg of brolucizumab experienced a mean gain in BCVA of 6.9 letters compared with 7.6 letters in the aflibercept group. Additionally, 57 percent of HAWK patients and 52 percent of HARRIER patients who received 6 mg of brolucizumab were maintained on a 12-week interval after the loading phase until the final end point of week 48.
Another exciting anti-VEGF drug is conbercept, which is made by Kang Hong Biotech from China. “It may be the first drug that’s FDA-approved from China,” Dr. Dugel explains. “It’s a fusion protein with a change in domain IV that will potentially allow it to bind better to the target than we’ve seen before.”
A study was conducted to compare the efficacy and safety of conbercept and ranibizumab (Lucentis, Genentech) when administered according to a treat-and-extend (TREX) protocol for the treatment of neovascular AMD in China.2 The study concluded that both drugs had equivalent effects in visual and anatomic gains at one year. Longer treatment intervals were achieved in more patients in the conbercept group.
Allergan’s abicipar pegol is also showing promise. “It’s a very small drug that theoretically has great potential and may have greater durability than any of the drugs that we’ve had so far,” says Dr. Dugel. “However, the challenge for this drug is that the inflammation rate is very high, 15 percent, so we’re hoping to get more clarity on that.”
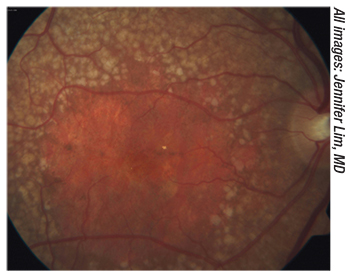 |
| Figure 1. New drugs may slow the progression of geographic atrophy. |
In the Phase II trial, abicipar demonstrated durability of effect.3 Best-corrected acuity and central retinal thickness (CRT) improvements were similar between abicipar and ranibizumab at weeks 16 and 20 (eight and 12 weeks after the last abicipar injection and four weeks after the last ranibizumab injection). No serious adverse events were reported.
This multicenter, randomized, double-masked comparison included 64 patients who received intravitreal injections of abicipar 1 mg or 2 mg at baseline, week four, and week eight (three injections) or ranibizumab 0.5 mg at baseline and monthly for five months.
Twenty-five patients received 1 mg of abicipar, 23 patients received 2 mg of abicipar, and 16 patients received ranibizumab. Least-squares mean BCVA change from baseline was +6.2, +8.3 and +5.6 letters at week 16 and +8.2, +10 and +5.3 letters at week 20. Least-squares mean CRT reduction from baseline was 134, 113, and 131 µm at week 16, and 116, 103 and 138 µm, respectively, at week 20. Five of 48 patients who were treated with abicipar experienced intraocular inflammation, which resolved without sustained vision loss.
Combination Drugs
Several combination drugs are under development. Topping the list is faricimab, which used to be known as RG7716, from Genentech/Roche. “This is a bispecific antibody, so it will simultaneously bind to Ang-2 and VEGF-A,” Dr. Dugel says. “The studies that have been done in neovascular AMD and DME have been very encouraging.”
“This dual mechanism of action is attractive in that it provides inhibition of the neovascular AMD process via two separate pathways,” says Chicago’s Jennifer Lim, MD.
The Phase II STAIRWAY study explored the extended durability of faricimab in the treatment of wet AMD.4 At 52 weeks, faricimab patients dosed either every 16 weeks or every 12 weeks demonstrated sustained vision outcomes comparable to ranibizumab dosed every four weeks.
This study assessed two extended-dosing regimens of faricimab 6 mg given every 16 weeks or every 12 weeks, compared to ranibizumab 0.5 mg every four weeks. At week 24, which was three months after the last loading dose, patients who received faricimab every 16 weeks were switched to 12-week dosing if they were shown to have active disease, per predefined criteria. At week 24, 65 percent of patients treated with faricimab had no active disease, demonstrating the potential of 16-week dosing in nearly two-thirds of patients. Initial gains in BCVA were fully maintained through week 52 with 16- and 12-week dosing regimens.
Patients treated with faricimab dosed every 16 weeks experienced a mean improvement of 11.4 letters from baseline, compared to 10.1 letters in patients treated with faricimab dosed every 12 weeks and 9.6 letters in patients treated with ranibizumab 0.5 mg dosed every four weeks. Comparable reductions in CRT were also noted in patients treated with both dosing intervals of faricimab and those treated with ranibizumab. In STAIRWAY, the rates of ocular and systemic adverse events observed with faricimab were similar to those with ranibizumab. “These results are very encouraging given the less frequent dosing interval of faricimab,” Dr. Lim says.
Based on these data, Genentech/Roche will be initiating a global Phase III program for faricimab in wet AMD. “The LUCERNE Study (Phase III faricimab versus aflibercept) is unique in its design in that it will explore different dosing intervals in the first year and then a ‘personalized treatment interval’ from week 60 onward,” Dr. Lim says. “This will be of great value for the day-to-day care of patients if the drug is approved.”
Another promising drug is OPT-302 from Opthea, an Australian company. “It suppresses VEGF-C and D,” Dr. Dugel explains. “It would allow for a pan-VEGF inhibition in combination with any of the VEGF-A inhibitors currently available. The early studies have been very encouraging, and the Phase III studies are being done in neovascular AMD and DME.”
There is also the drug previously known as Luminate. It’s now called risuteganib from Allegro. “The results have been encouraging for diabetic macular edema,” Dr. Dugel says.
Last is a topical agent from Oculus that contains a cycloheximide platform. It’s currently being studied in Scandinavia and Eastern Europe, according to Dr. Dugel.
Dry AMD/Geographic Atrophy
Apellis has developed a drug to treat geographic atrophy called APL-2, which is a complement inhibitor. “It inhibits the transformation of C3, and the preliminary results have been very encouraging in slowing down the progression of geographic atrophy,” Dr. Dugel says. “However, the downside is that there seems to be an increased risk of developing neovascular macular degeneration. We don’t know why yet, but Phase III studies are under way. This is the first time that we’ve seen something that shows an encouraging result in geographic atrophy.”
Dr. Lim emphasizes that more data will be necessary to make a decision about it. “This increased risk of CNV may prove to be the limiting factor in the use of this drug,” she says.
According to Apellis, APL-2 is designed to inhibit the complement cascade centrally at C3, and it may have the potential to treat a wide range of complement-mediated diseases more effectively than is possible with partial inhibitors of complement.5 APL-2 is a synthetic cyclic peptide conjugated to a polyethylene glycol (PEG) polymer that binds specifically to C3 and C3b, effectively blocking all three pathways of complement activation. Interim data from three trials have demonstrated meaningful improvements in lactate dehydrogenase and hemoglobin levels in previously untreated patients and in patients who are suboptimal responders to eculizumab.
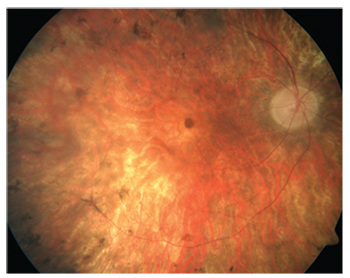 |
| Figure 2. Gene therapy has finally provided a treatment for a form of retinitis pigmentosa. |
Another interesting drug is AKB-9778 from Aerpio. “It is currently being studied for diabetic retinopathy,” Dr. Dugel says. “It’s delivered subcutaneously, so it goes to both eyes systemically. In previous studies it was associated with a decrease in progression of diabetic retinopathy, as well as improvement in renal function. If any of these results are duplicated in this study, I think it will have an enormous impact, not just because it’s a different mechanism of action but also because of its subcutaneous delivery.”
The Phase IIa clinical trial found that activation of Tie2 by subcutaneous injections of AKB-9778 combined with suppression of vascular endothelial growth factor caused a significantly greater reduction in DME than that seen with suppression of VEGF alone, and similar reductions in the two-step progression of diabetic retinopathy were seen compared to ranibizumab in both eyes.6
Gene Therapy
Luxturna (voretigene neparvovec) from Spark is the first gene therapy in ophthalmology to be FDA-approved. It’s a gene replacement for RPE65 for patients with retinitis pigmentosa, as well as Leber’s. “It’s a groundbreaking, unique treatment that gets to the heart of the cause of the problem for this type of retinitis pigmentosa,” says Dr. Lim. “I think it gives patients hope where there wasn’t any hope before. Because these children can now be cured, they won’t be relegated to a lifetime of blindness.”
Phase I studies showed potential benefit of gene replacement in RPE65-mediated inherited retinal dystrophy. The Phase III study found that voretigene neparvovec gene replacement improved functional vision in RPE65-mediated inherited retinal dystrophy, previously medically untreatable.7
The open-label, randomized, controlled Phase III trial was conducted at two sites in the United States in patients aged 3 years or older with BCVA of 20/60 or worse and/or visual field less than 20 degrees in any meridian, or both, in both eyes, with confirmed genetic diagnosis of biallelic RPE65 mutations, sufficient viable retina, and the ability to perform standardized multi-luminance mobility testing (MLMT) within the luminance range evaluated. Participants were randomly assigned (2:1) to intervention or control. Intervention was bilateral, subretinal injection of 1.5 × 1011 vector genomes of voretigene neparvovec, 0.3 mL total volume. The primary efficacy endpoint was the one-year change in MLMT performance, measuring functional vision at specified light levels. The intention-to-treat and modified ITT populations were included in primary and safety analyses.
The study included 29 patients who were randomly assigned to intervention (n=20) or control (n=9). At one year, mean bilateral MLMT change score was 1.8 light levels in the intervention group versus 0.2 in the control group. Thirteen (65 percent) of 20 intervention participants, but no control participants, passed MLMT at the lowest luminance level tested (1 lux), demonstrating maximum possible improvement.
For treating chronic disease, RegenXBio has developed RGX-314, which is being developed as a one-time subretinal treatment for wet AMD. It includes the NAV AAV8 vector encoding an antibody fragment, which inhibits VEGF, modifying the pathway for formation of new leaky blood vessels that lead to retinal fluid accumulation and vision loss. In preclinical animal models with conditions similar to macular degeneration, significant and dose-dependent reduction of blood vessel growth and prevention of disease progression was observed after a single subretinal dose of RGX-314.8
“This is an actual anti-VEGF Fab gene put in a specialized virus vector, which is technologically very difficult,” Dr. Dugel explains. “In other words, it’s not just a generic virus vector, which is usually AAV2; it’s AAV8, which is specialized to go specifically to the retina. And they’ve been able to show, for the first time, that there is a dose relationship with expression of the protein, which is extraordinarily important. It’s the first time we’ve ever seen that, and the early phase study has been very encouraging. With a higher dose, there’s a dose relationship, and there’s a decrease in the number of injections and an increase in the visual acuity. But to me, the most important thing is that there is an objective dose relationship in terms of the protein that’s produced. I look forward to further results, but also to the next phase in study. If that finding is duplicated in a larger study, it will transition gene therapy from a rare disease treatment into a chronic disease treatment. That will really change everything we do.”
The Phase I clinical trial has enrolled 24 previously treated wet AMD patients who are responsive to anti-VEGF therapy and are 50 years of age or older in four cohorts.8 The study is designed to evaluate four doses of RGX-314: 3 x 109 GC/eye; 1 x 1010 GC/eye; 6 x 1010 GC/eye; and 1.6 x 1011 GC/eye. The study will evaluate the safety and tolerability of RGX-314 at 24 weeks after a single dose administered subretinally. Primary endpoints include safety and tolerability. Following completion of the primary study period, patients will continue to be assessed until week 106 for long-term safety and durability of effect.
Dr. Lim is optimistic about the future of retinal therapies. “The future is bright for patients with neovascular AMD and even rarer genetic diseases such as RPE65 RP,” she says. “New drugs with longer duration of action, different molecular targets, combination therapies and even extended drug delivery may be on the horizon. For non-neovascular AMD and geographic atrophy, advances are also occurring. Innovation and scientific discovery will hopefully alleviate the suffering of many of our patients in the near future.” REVIEW
Dr. Dugel is on the scientific advisory board of Novartis, Genentech, Aerpio and Opthea. He is a consultant to Genentech, Novartis, Allergan, Aerpio, Opthea, Spark, Regeneron and Chendu Kang Hong Biotechnology, and he is a minor stockholder in Aerpio. Dr. Lim has a financial interest in Genentech, Regeneron and Aerpio.
1. Phase 3 Randomized, Double-Masked studies of brolucizumab versus aflibercept in nAMD: Expanded primary and secondary outcomes from HAWK/HARRIER. Presented at: American Society of Retina Specialists annual meeting; July 20-25, 2018; Vancouver, British Columbia.
2. Cui J, Sun D, Lu H, et al. Comparison of effectiveness and safety between conbercept and ranibizumab for treatment of neovascular age-related macular degeneration. A retrospective case-controlled non-inferiority multiple center study. Eye (Lond) 2018;32:2:391-399.
3. Callanan D, Kunimoto D, Maturi RK, et al. Double-masked, randomized, phase 2 evaluation of abicipar pegol (an anti-VEGF DARPin therapeutic) in neovascular age-related macular degeneration. J Ocul Pharmacol Ther. 2018. [Epub ahead of print]
4. Genentech press release. https://www.gene.com/media/press-releases/14762/2018-10-27/new-stairway-study-data-shows-potential-. Accessed 19 February 2019.
5. Apellis press release. http://investors.apellis.com/news-releases/news-release-details/apellis-finalizes-phase-3-clinical-trial-plans-geographic. Accessed 19 February 2019.
6. Campochiaro PA, Khanani A, Singer M, et al. Enhanced benefit in diabetic macular edema from AKB-9778 Tie2 activation combined with vascular endothelial growth factor suppression. Ophthalmology 2016;123:8:1722-1730.
7. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomized, controlled, open-label, phase 3 trial. Lancet 2017;390_10097:849-860.
8. Regenxbio press release. https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-additional-positive-interim-phase-i-trial. Accessed 19 February 2019.



