Some surgeons consider the femtosecond laser to be the most disruptive technological contribution to cataract surgery in recent years.1 Many surgeons rely on it during the formation of LASIK flaps and for capsulotomy creation. At the 2018 American Society of Cataract and Refractive Surgery meeting in Washington, D.C., three manufacturers announced new 510(k) clearances from the U.S. Food and Drug Administration that enable their femto systems to support presbyopia-correcting procedures, facilitate keratoconus treatments and address astigmatism. The manufacturers say that they’re responding to a growing patient demand for treatments beyond refractive and cataract procedures that will aid spectacle independence, such as corneal inlays and intracorneal ring segments. Here’s a rundown of what’s new.
LenSx
The LenSx femtosecond laser platform (Alcon; Fort Worth, Texas), first made commercially available in 2011, was the first in the category to receive FDA approval. In late March, the laser gained two new indications: the creation of tunnels for the placement of corneal rings; and the
 |
| Updates to the LenSx include corneal pocket- and tunnel-cutting capabilities. |
creation of corneal pockets for the placement of presbyopia-correcting inlays. The company also says that new features include an enhanced graphical interface and updated software. The LenSx system will also be available with gold or white exterior skin colors to help color-coordinate the surgical suite.
Michael Lawless, MBBS, FRANZCO, of Vision Eye Institute, Chatswood, New South Wales, and a clinical associate professor at Sydney Medical School in Australia, notes that although he doesn’t commonly implant corneal rings in his practice,“it’s helpful to have this capability. It will be available with the release of the new software.”
Elizabeth Yeu, MD, partner at Virginia Eye Consultants and an assistant professor at Eastern Virginia Medical School in Norfolk, welcomes the changes coming to the LenSx, “including created greater automation of the steps and improved efficiencies. This newest change provides expanded capabilities to the system, including corneal flaps and tunnels; and the imaging upgrade allows for more precise placement of our cataract surgical wounds,” she says. “While corneal refractive surgery can be performed in either an office-based laser suite or in the OR, this update provides an alternative option so surgeons can perform LASIK, corneal inlays and ring segments in the OR setting.”
VisuMax
In September 2016 the VisuMax femtosecond laser system (Carl Zeiss Meditec; Jena, Germany) got FDA
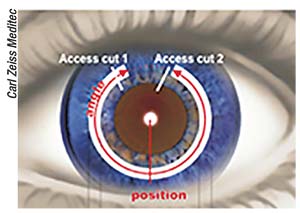 |
| Zeiss says VisuMax can cut tunnels to fit any ring dimensions, in arcs between 90 and 270 degrees or a full 360-degree arc. |
approval for small-incision lenticule extraction, which enables surgeons to correct myopia without stromal ablation or flap-cutting. In time for ASCRS 2018, Zeiss announced that the VisuMax has received 510(k) clearance for its new corneal tunnel-cutting options, allowing surgeons to control many aspects of corneal ring placement for the treatment of keratoconus or astigmatism.
The company says that the VisuMax has the most flexible software for the creation of intracorneal ring tunnels, allowing surgeon adjustment of numerous parameters that can shape the clinical effect of ICRs. “The user chooses the tunnel width, diameter, depth and length in accordance with the intracorneal ring parameters and surgeon preference. These parameters are then saved as preferences for repeating procedures,” explains Steve Schallhorn, MD, Zeiss’ chief medical officer for global ophthalmic devices. “The ICR tunnels created by the VisuMax are very high precision, and are tailored to the surgeon’s preferences.”
Surgeons can select partial corneal-tunnel incisions of 90 to 270 degrees, as well as full 360-degree arcs. The manufacturer claims that the VisuMax can be set to cut tunnels for any intracorneal ring dimension to control how tightly the ring or ring segment will fit. The user can also program the VisuMax to cut intracorneal ring tunnels at a tilt based on the outer ring diameter and the chosen depth of implantation. The ability to choose tunnel access cut width, shape and opening angle helps enhance patient comfort and prevent tissue tearing during ring insertion, according to Zeiss. The surgeon can choose to make zero, one or two access cuts; and the VisuMax supports surgeon preference for manual ring implantation.
Lensar
The Lensar femtosecond laser system with Streamline IV (Lensar; Orlando, Florida) also gained new FDA-cleared indications for corneal-pocket cuts and incisions. Lensar says the accompanying system upgrades expand the Lensar’s FDA-approved capabilities to support surgical presbyopia correction. The Windows operating system has also been updated from Windows 7 to Windows 10. A new Patient Interface Device (PID) Kit for use during pocket and flap cutting consists of a curved contact lens attached to the suction ring for enhanced stability. As an alternative to sterilization of the PID Kit using gamma radiation, Lensar has added the option of ethylene-oxide sterilization.
The company’s Streamline IV platform for pocket cuts includes a drop-down menu that allows the surgeon to choose pocket, flap or flocket settings. The surgeon can then enter a value for pocket depth, depending on the inlay device being used, to create a stromal pocket of the FDA-recommended depth for that device.
The Lensar already provides precision control over the size of the capsular opening, the type and
 |
parameters of laser fragmentation treatment within the lens, and the size, architecture and location of full- and partial-thickness corneal incisions. The newly approved indication adds control of the size, architecture and depth of corneal pocket and flap cuts.
“Adapting the Lensar platform for stromal pockets and corneal flaps delivers on the company’s promise to expand the platform capabilities to facilitate presbyopia inlay procedures,” says Gregory Parkhurst, MD, FACS, of Parkhurst NuVision in San Antonio. “With more and more patients considering surgical alternatives to glasses and contacts to correct their near-vision issues, being able to leverage the features of the Lensar femtosecond platform for corneal inlays is a real benefit to surgeons.”
Lensar has also applied for CE approval of the new pocket-cutting features in the EU, and the FDA-cleared features will be made available to U.S. surgeons this year. REVIEW
Dr. Lawless and Dr. Yeu are consultants for Alcon. Dr. Parkhurst is a consultant for Lensar, Alcon and Carl Zeiss Meditec.
1. Roberts TV, Lawless M, Sutton G, Hodge C. Update and clinical utility of the LenSx femtosecond laser in cataract surgery. Clin Ophthalmol 2016; 10: 2021–2029.



