Although primary open-angle glaucoma is more common than primary angle-closure glaucoma, the latter has higher visual morbidity, including blindness. Precursor stages of primary angle-closure glaucoma are characterized by iridotrabecular contact of at least 180 degrees of the angle, which can be detected with gonioscopy.
Unfortunately, angle-closure disease is often missed. Most patients with this condition don’t have symptomatic attacks of angle closure that might prompt them to seek eye care. Even when a patient presents for an eye exam, gonioscopy may not al-ways be performed. The van Herick test, which estimates the peripheral anterior chamber depth relative to the peripheral corneal thickness, is commonly used to decide which patient should undergo gonioscopy. The most significant problem with this approach is that it doesn’t have a high enough sensitivity and specificity to catch all patients with angle closure. In one study, the van Herick test, per-formed by technicians, residents and attendings, was compared to gonioscopy performed by attendings as the gold standard; the sensitivity of the van Herick test to detect angle closure was only 58 to 79 percent.1
Gonioscopy Pros and Cons
Gonioscopy is the gold standard for diagnosing angle closure. As a glaucoma specialist, I specifically check the angle with gonioscopy on a regular basis. It does require an anesthetic, but it usually takes less than a minute. In addition to providing a quick 360-degree assessment of the angle width—with and without indentation—peripheral anterior synechiae can be detected, the degree of trabecular meshwork pigmentation can be observed, and abnormalities such as neovascularization of the angle and angle recession can be diagnosed.
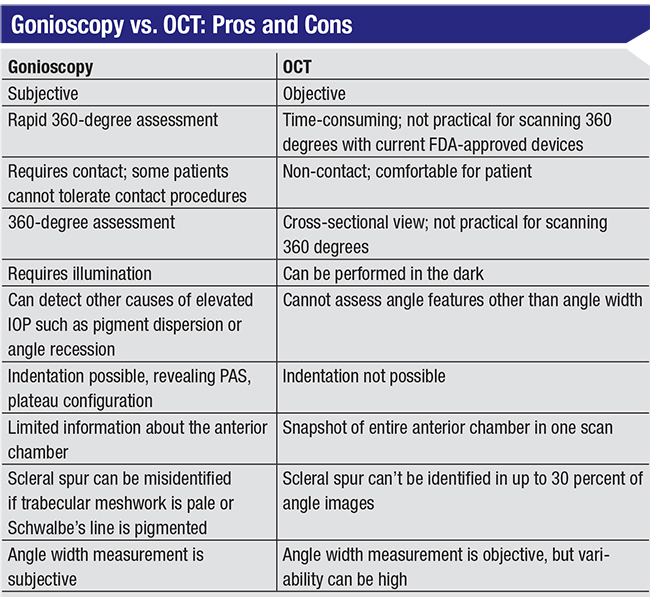
However, gonioscopy has some limitations. It’s a subjective technique that requires contact with the eye and slit lamp illumination, both of which can artificially widen the angle. It’s also not performed as of-ten as recommended. One reason for this may be that in clinics where technicians perform the initial slit lamp assessment and then dilate the patient, making the patient wait for gonioscopy by the ophthalmologist might be considered disruptive to the schedule. Furthermore, it’s possible to misdiagnose the angle status, even when you perform gonioscopy—especially if you don’t do it very often. For example, a pigmented Schwalbe’s line might be mistaken for the trabecular meshwork, creating the erroneous impression of an open angle when the angle is actually closed. Similarly, if the trabecular meshwork is very pale, it can be difficult to determine the condition of the angle structures. There are some workarounds to help with this, such as the corneal wedge technique that can help you identify Schwalbe’s line, but the fact is, sometimes even with gonioscopy it can be difficult to tell what’s going on in the angle. (Of course, the more you do it, and the more familiar you are with these issues, the more likely you are to make an accurate assessment.)
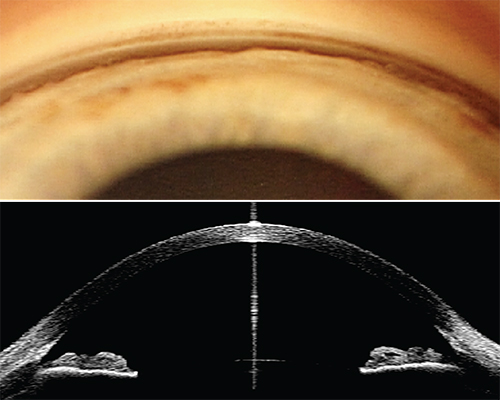 |
| Both gonioscopy and anterior-segment optical coherence tomography have pros and cons when it comes to assessing the condition of the angle. |
Weighing the Alternatives
These considerations—and the reality that many cases of closed angles are not being caught—open the door to other ways of assessing the angle that might bypass some of gonioscopy’s limitations. These include:
• The scanning peripheral depth analyzer. This instrument (not available in the United States) uses visible light to automatically scan from the optical axis to the limbus, estimating the distance between the cornea and the iris and quantifying it into different grades. It’s an easy-to-use, non-contact method to indirectly estimate the angle width, but the technology doesn’t have high enough specificity to be a good alternative to gonioscopy.2,3
• The Pentacam. The Pentacam uses a rotating Scheimpflug camera and provides cross-sectional images of the anterior chamber, but it’s not able to visualize the angle recess itself. Instead, it makes some extrapolations based on the corneal surface and iris surface to draw conclusions about the angle recess.
• Ultrasound biomicroscopy. UBM uses high-frequency ultrasound and gives excellent cross-sectional views of the angle structures, including the ciliary body. However, it’s cumbersome to use on a routine basis and requires a highly skilled operator to perform the scans. Those considerations make it unsuitable for use as a screening tool for angle closure.
Anterior segment OCT is another cross-sectional imaging modality that can visualize the anterior chamber and angle structures. However, unlike UBM, OCT is an optical technology; it can’t penetrate the iris pigment epithelium, so the ciliary body can’t be viewed. The Visante OCT (Carl Zeiss Meditec) uses a wavelength of 1310 µm, which is ideal for visualizing angle structures; but, unfortunately, the Visante is no longer being manufactured. Retina-focused OCT instruments that are widely available, such as Carl Zeiss Meditec’s Cirrus, Heidelberg’s Spectralis and Optovue’s Avanti, can also be used to image the anterior segment, but these use light wavelengths between 800 and 900 µm that don’t penetrate the angle as well. As a result, the images are less detailed in the angle region than those produced by the Visante OCT. Nevertheless, it’s usually possible to tell whether the iris and cornea are touching.
Probably the primary reasons these instruments are seldom used for angle analysis is because of workflow in the clinic. They’re normally used after the patient has been dilated, and they usually require an additional external lens for imaging the front of the eye. Nevertheless, I have seen some doctors use retinal OCT to assess the angle.
 |
The AS-OCT Advantage
Although gonioscopy is the gold standard for angle assessment, a noncontact technology that could image the angle in the dark without illumination would, in theory, allow us to screen patients much more easily. In fact, when anterior segment OCT was first developed, it was seen as a very promising way to screen for angle closure.
This approach has some clear potential advantages over gonioscopy. Those include:
• It’s easy to use. OCT doesn’t require an ophthalmologist with expertise in performing gonioscopy to diagnose iridotrabecular contact.
• It’s comfortable for the patient. In contrast, gonioscopy requires touching the eye, which patients generally prefer to avoid.
• It may produce fewer artifacts. As mentioned earlier, to perform gonioscopy you need slit lamp illumination and you have to touch the eye, both of which can artificially widen the angle. With OCT there’s no need to touch the eye, and because it can be done in the dark it reveals the physiological state of the angle, giving us a better sense of the true condition of the angle.
• It can quantify angle width and changes occurring with pupil dilation. Another useful test related to angle closure is seeing how the angle opens (or closes) as the pupil reacts to greater or lesser illumination. Both gonioscopy and OCT can be used to evaluate this, but OCT allows us to quantify the amount of change.
• OCT detects more angle closure than gonioscopy. Ironically, one reason that OCT has low specificity when used to assess the angle in studies is that it actually detects more angle closure than gonioscopy, which is treated as the gold standard for comparison.
Being able to detect more angle closure than gonioscopy can certainly be seen as a good thing. However, angle closure on OCT doesn’t al-ways mean that the patient should immediately be treated. In fact, even patients with gonioscopic angle closure don’t always require treatment. In the case of primary angle closure suspects who are usually asymptomatic and have no abnormality other than angle closure, the American Academy of Ophthalmology’s Preferred Practice Patterns leaves decisions about treatment up to the doctor, saying that treatment with iridotomy in this situation “may be considered.” Therefore, using OCT alone isn’t sufficient to decide how a patient should be treated. However, it can help to identify individuals who should be checked more carefully for a problem in the future.
• It may detect the likelihood of future angle closure better than gonioscopy. Interestingly, one study looked at patients who were classified as having open angles by gonioscopy, but classified as having closed angles with OCT.4 The study found that after four years, 17 percent of the patients in this category that returned for follow-up had developed closed angles in two or more quadrants (as classified by gonioscopy), and 10 percent developed angle closure in three or more quadrants.
Although this finding was based on only the 62 percent of the original patient sample that returned for follow-up at year four, this is significant. On gonioscopy, these people appeared to have open angles. Yet some went on to develop closed angles, and OCT picked up on that be-fore closure was visible on gonioscopy. If a patient appears to have open angles, most ophthalmologists won’t be checking periodically to see if the angle is closing. That leaves these individuals open to future trouble—trouble that might be prevented by an OCT scan.
Ironically, this potential advantage to scanning with anterior segment OCT wouldn’t be particularly useful to me, because as a glaucoma specialist, I perform gonioscopy on all of my patients on a regular basis—even if the angle has appeared to be open in the past. If all seems well, I check them every three or four years; if they’re borderline, I check them every year. However, if OCT is able to catch some patients at risk for future trouble, patients that gonioscopy isn’t able to identify, this could be useful for a doctor who doesn’t perform gonioscopy very often. It might pro-vide a warning that this patient needs to be checked periodically, despite the appearance of an open angle. (That could mean referring the patient to a glaucoma specialist who’s more comfortable with regular gonioscopy screening.)
OCT Disadvantages
Despite its advantages, at a practical level in the clinic, using OCT as an alternative for examining the condition of the angle can be problematic, for a number of reasons:
• Anterior segment OCT isn’t widely available. That means the equipment most appropriate for evaluating the angle isn’t available in many practices.
• Most OCT scans (used for evaluating the optic nerve and retina) are performed after the patient has been dilated. Even if you decide to use OCT technology that’s not optimized for viewing anterior segment structures, this use may not be convenient in terms of patient flow.
| Even when relying primarily on gonioscopy to assess the angle, AS-OCT serves several very useful purposes. |
• OCT only measures a tiny fraction of the angle. Primary angle closure is defined in the Academy’s Preferred Practice Patterns as being characterized by iridotrabecular contact for at least 180 degrees. (Iridotrabecular contact is defined as the posterior trabecular meshwork not being visible using static gonioscopy.) This is relevant when comparing OCT and gonioscopy, because OCT typically only scans a slice from four quadrants. Essentially, you’re seeing the condition of the angle in four degrees out of 360.
Since the definition of angle closure is iridotrabecular contact over at least 180 degrees, we conclude that if two of four OCT scans show a closed angle, that indicates at least 180 degrees of closure. (Trying to base the conclusion on a larger number of scans can be problematic. Among other issues, scanning the superior and inferior quadrants is challenging because the eyelid gets in the way.) In contrast, gonioscopy provides in-formation about the entire angle.
Of note, there is a new swept-source, 1310-µm OCT instrument not approved in the United States that does allow 360-degree measurement of the angle. So far, there’s no data to suggest that this technology is superior to gonioscopy, but in theory it might be a viable alternative for detecting angle closure. (However, even where this technology is available, it doesn’t appear to be replacing gonioscopy.)
• Quantifying the angle requires subjective input about the location of the scleral spur. The scleral spur can be difficult to identify with OCT in up to 30 percent of images. This is a problem if you’re trying to quantify the opening of the angle using OCT; the person doing the measurement has to decide where the scleral spur is, resulting in variability in the outcome depending on that decision. In addition, when assessing change over time, one can’t be sure that the same region is being scanned, and measurement variability can be introduced due to differences in the iris structure at different locations. For these reasons, I don’t routinely use OCT for quantitative analysis of the angle.
Population-based Screening
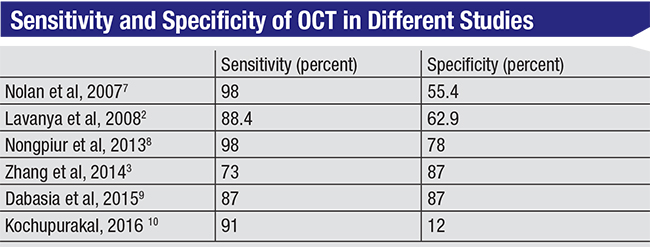
One important concern is that we’d like to have an instrument that can easily act as a screening tool in the general population to find individuals with angle closure. Aside from needing a technology that’s simple to use, a major consideration here is having high specificity in order to avoid too many false positives. Generally, a specificity of 95 to 98 percent has been recommended for screening, along with a sensitivity of at least 85 percent.5,6 If a technology has high sensitivity and low specificity, it will flag most patients as having a closed angle, including many who don’t actually have it, burdening the health-care system.
This is an area in which OCT doesn’t do well. Studies of OCT used for detection of angle closure have shown widely varying results in comparison to gonioscopy, with specificity ranging from 12 to 87 percent—although it’s important to note that the definition of angle closure isn’t uniform across the studies. In addition, finding angle closure on OCT doesn’t always imply the need for immediate treatment. Given these facts, I don’t believe OCT can be justified as a screening tool for angle closure out in the community.
OCT’s usefulness as a screening tool does increase, however, if the screening is being done in the office (i.e., opportunistic screening) rather than out in the world, especially in settings where gonioscopy may not be feasible. In the clinic, a higher false-positive rate may be acceptable. In that environment, high sensitivity—a limited number of false negatives—is more important. However, in this setting the cost and availability of OCT is a limitation. If there’s adequate expertise, gonioscopy is a faster, cheaper, way to assess the angle and make treatment decisions in a patient who is already in the clinic.
OCT as an Adjunct
In my experience, the approach that’s most effective for angle assessment is to use gonioscopy as the primary tool with anterior segment OCT reserved as an adjunct technology. Even when relying primarily on gonioscopy to assess the angle, AS-OCT serves several very useful purposes:
 |
• OCT can help to determine the cause of the angle closure. OCT is useful for identifying the mechanism(s) behind the angle closure. The various mechanisms of primary angle closure often coexist; they include pupillary block, high lens vault, thick peripheral iris, and an anteriorly positioned ciliary body (although the last can’t be seen with OCT—only with ultrasound biomicroscopy). OCT can also reveal causes of secondary angle closure, such as supraciliary fluid that’s pushing the iris-lens diaphragm forward. It’s important to know this because the appropriate treatment varies depending upon the cause of the problem.
• OCT is excellent for patient education. In my practice, people often come in for a second opinion about whether they should receive a laser iridotomy. In most cases they’re totally asymptomatic; a general ophthalmologist or an optometrist has noted their closed angle and suggested the iridotomy. Patients find that idea scary when they haven’t heard of it before, so they come to a glaucoma specialist to find out if this is correct.
Of course, I could show them a drawing or generic photo of a normal angle versus a closed angle to help them understand the problem, but I’ve found that showing the patient a picture of his or her own angle compared to an open angle is most effective. Then I can explain how angle closure attacks can happen and how an iridotomy might help.
• OCT can be helpful in situations where gonioscopy isn’t feasible. Occasionally a patient will present with a hazy cornea, making angle visualization difficult or impossible; OCT can still provide a view of the angle. Also, some patients are unable to tolerate a contact lens placed on the eye. OCT can allow angle evaluation without touching the eye.
In the final analysis, OCT is a great adjunct, but there’s no substitute for performing gonioscopy. REVIEW
Dr. Radhakrishnan is a glaucoma specialist at the Glaucoma Center of San Francisco and research di-rector at the Glaucoma Research and Education Group, also in San Francisco. She reports no relevant financial disclosures.
1. Johnson TV, Ramulu PY, Quigley HA, Singman EL. Low sensitivity of the Van Herick method for detecting gonioscopic angle closure independent of observer expertise. Am J Ophthalmol 2018;195:63-71.
2. Lavanya R, Foster PJ, Sakata LM, et al. Screening for narrow angles in the Singapore population: Evaluation of new noncontact screening methods. Ophthalmology 2008;115:10:1720-7, 1727.e1-2.
3. Zhang Y, Li SZ, Li L, Thomas R, Wang NL. The Handan Eye Study: Comparison of screening methods for primary angle closure suspects in a rural Chinese population. Ophthalmic Epidemiol 2014;21:4:268-75.
4. Baskaran M, Iyer JV, Narayanaswamy AK, et al. Anterior segment imaging predicts incident gonioscopic angle closure. Ophthalmology 2015;122:12:2380-4.
5. Stamper RL. Glaucoma Screening. J Glaucoma 1998;7:3:149-50.
6. Thomas R, Parikh R, Paul P, Muliyil J. Population-based screening versus case detection. Indian J Ophthalmol 2002;50:3:233-7.
7. Nolan WP, See JL, Chew PT, et al. Detection of primary angle closure using anterior segment optical coherence tomography in Asian eyes. Ophthalmology 2007;114:1:33-9.
8. Nongpiur ME, Haaland BA, Friedman DS, et al. Classification algorithms based on anterior segment optical coherence tomography measurements for detection of angle closure. Ophthalmology 2013;120:1:48-54.
9. Dabasia PL, Edgar DF, Murdoch IE, Lawrenson JG. Noncontact screening methods for the detection of narrow anterior chamber angles. Invest Ophthalmol Vis Sci 2015;56:6:3929-35.
10. Kochupurakal RT. Role of optical coherence tomography in assessing anterior chamber angles. J Clin Diagn Res. 2016;10:4:NC18–NC20.



