Diquafosol tetrasodium is a P2Y2 receptor agonist that stimulates fluid and mucin secretion on the ocular surface. A research group of American investigators from four states says this novel topical treatment for dry eye is well-tolerated and superior to placebo in reducing corneal staining and in relieving certain patient symptoms.
In this randomized, double-masked, parallel group, placebo-controlled trial funded by Inspire Pharmaceuticals (Durham, N.C.), investigators sought to evaluate the safety and efficacy of two dose strengths of diquafosol (1% and 2%) relative to placebo (vehicle alone) in subjects with dry-eye disease.
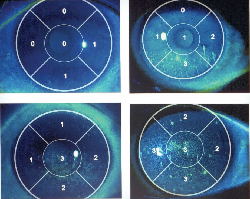 |
| Fluorescein grading scale as used in this clinical trial of Diquafosol tetrasodium. Images: J. Tauber, MD. |
At 33 U.S. sites between March and December 2001, researchers studied 527 patients randomized for treatment. The 2% diquafosol group included 175 patients, while 176 were in the 1% diquafosol group, and 176 in the placebo group. Eighty-nine percent of subjects completed the 24-week study, and withdrawals were comparable across all treatment groups. The majority of subjects were female, Caucasian and elderly, reporting their "worst" dry-eye symptom to be foreign body sensation.
Corneal staining scores of the 2% diquafosol group were superior to placebo (p<0.001) at the six-week endpoint, and maintained superiority at 12 weeks and 24 weeks. Subjects in the 1% diquafosol group also were superior to placebo with respect to corneal staining scores at the six-week endpoint (p=0.002).
Considering the primary subjective variable, foreign body sensation, significant differences between the 2% diquafosol group and placebo were not achieved until the six-week endpoint (p<0.05) and continued in weeks eight and 10.
Researchers point out that one week after cessation of therapy, the beneficial effects of diquafosol with respect to corneal staining were diminished, suggesting the need for ongoing pharmacologic therapy.
(Cornea 2004;23:784-792)
Tauber J, Davitt WF, Bokosky JE, Nichols KK, Yerxa BR, Schaberg AE, LaVange LM, Mills-Wilson MC, Kellerman DJ
Contrast Sensitivity Improves with Phakic Lens
Implantation of the Verisyse toric iris-claw lens (AMO) in phakic eyes to correct high or moderate myopia with astigmatism has the potential to improve contrast sensitivity, say researchers based on their German study.
The study doctors tested contrast sensitivity at spatial frequencies of 3, 6, 12 and 18 cycles per degree before surgery and three months postop. Patients were aged 18 years or older with a mean preop spherical equivalent of
-12.65 ±3.6 D. All had astigmatism greater than 1.5 D.
Postop there was an increase in mean contrast sensitivity at all spatial frequencies. The differences were statistically significant only at 6 cpd (p=.033) and 12 cpd (p=.032).
Researchers propose that no loss of contrast sensitivity after iris-claw IOL implantation might be explained by the preservation of corneal integrity (unlike LASIK, PRK, etc.) and the subsequent minimization of induced optical aberrations.
(J Cataract Refract Surg 2004;30:2284-2289)
Dick HB, Tehrani M, Aliyeva S




