|
In this article, surgeons discuss the concept of dysfunctional lens syndrome, how they talk about it with patients and some of the controversial aspects of using it as a diagnosis.
The DLS Concept
Surgeons who use the DLS diagnosis say it clarifies a process that was often hazy in the minds of physicians and their patients.
“What I noticed before we started discussing DLS,” says Overland Park, Kan., surgeon Dan Durrie, “is that someone in his 40s, 50s, 60s or 70s would come to us with some vision changes and we’d say, ‘Good news, you don’t have a cataract.’ Then he’d come back every couple of years, each time noticing that his vision had gotten somewhat worse, and each time we’d send him off with, ‘You don’t have a cataract.’ And then, at some point, he’d come back and, miraculously, he would have a cataract that we could operate on because it had reached the point at which Medicare or insurance would cover it. In reality, the lens had been changing for 25 years.
“This way of communicating with patients about lens changes didn’t help them understand that they had a progressive problem that starts out with their ‘arms getting shorter’ in their 40s and ultimately ends in a cataract,” Dr. Durrie continues. “In their 40s, we’d tell them they had lost the zoom lens in their camera or were losing their accommodation and then, when they reached their 70s, we’d have a lot of discussion about cataract when they finally developed one, but there was this gap between age 43 to about 73—the average age people get cataract surgery—in which they didn’t really understand well what was going on with their lenses and what could be done about it.”
George Waring IV, MD, FACS, director of refractive surgery and associate professor of ophthalmology at the Medical University of South Carolina, Storm Eye Institute, also uses the dysfunctional lens diagnosis in his practice. “Years ago, for patients in their mid 50s or early 60s who complained of wearing their distance glasses, bifocals and/or contact lenses, we’d do LASIK,” he says. “And they’d be back in five years saying that their LASIK had worn off, but actually their lenses had aged. Because their lenses weren’t truly clear, this had a bearing on visual quality. This lack of clarity, however, wasn’t leading to symptoms that affected their daily activities or to an objective decrease in vision where the official diagnosis of cataract was warranted. So, instead of being enhanced with further LASIK, these patients would often go on to what was called clear lens extraction. However, we knew these weren’t clear lenses, and that the lens was the etiology of the problem. So, we began to use advanced techniques to study and characterize the lens changes that in the past had been dismissed as ‘pre-cataracts.’ ”
In DLS, then, the patient is made to understand that, barring a corneal or retinal issue, the presbyopia he experiences in his 40s, the slight haziness that comes on in the 50s and 60s and the eventual Medicare-defined cataract are all facets of the same thing, a dysfunctional lens.
Rather than confusing patients who are told they have a lens syndrome but it’s not covered by health insurance, Dr. Waring says the term DLS has actually helped. “The patient was coming in for LASIK and now you’re telling him you’re going to be removing his lens,” says Dr. Waring. “DLS has greatly facilitated what used to be a challenging discussion prior to the characterization of this spectrum of the aging lens’s changes. We now have advanced diagnostic and surgical instruments to evaluate and address changes in the crystalline lens. For example, femtosecond lasers are now available for lens surgery, like we have lasers for the cornea, and we can take patients on a digital tour of their eyes with advanced diagnostics to better educate them and to help in our clinical decision making. We can grade their visual quality and help them understand why it may make more sense for them to have laser surgery on their internal lens vs. their cornea—sparing them a second surgery in the process—and it’s been very well-accepted by patients.”
Presenting It to Patients
Surgeons who use the concept of DLS break it into three stages to help patients understand what’s going on.
• Stage I. Dr. Durrie says he begins by pulling out an eye model, slit-lamp photo, and/or an Eyemaginations digital presentation or computer diagram to highlight the crystalline lens for the patient. “I tell that patient that, in our 40s, the crystalline lens that was clear and pliable in our 20s begins to lose its ability to focus up close—or up close and in the distance for the hyperope—due to the lens getting stiffer and the muscles around it not being able to bend it as well,” Dr. Durrie says. “I say, ‘This is stage I of what you’re experiencing—dysfunctional lens syndrome. In stage I, the lens is still clear and colorless, but it’s stiffer, due to the disulfide bonds building up inside the lens, part of the glutathione pathway, which cross-links the lens fibers.’ I include the scientific detail at the end for inquisitive patients who want to know why it’s happening. It comforts the patient knowing that we understand what’s going on, and that it’s normal and every person gets it if he or she lives long enough.”
For patients in the first stage of DLS, besides glasses and contact lenses, there are three potential surgical options: monovision/blended vision; a presbyopic corneal inlay such as the Kamra (if the lens is clear); and, rarely at this stage, refractive lens exchange. “At this early stage, the last option would only be in someone such as a +2 or +4 D hyperope,” explains Dr. Durrie. “These patients are usually in their 40s and their lenses are still clear but have become dysfunctional. So, sometimes, rather than discussing other surgical options, we suggest that maybe a high hyperope be educated about the option of refractive lens exchange.”
• Stage II. Surgeons define this stage as occurring in the 50s and 60s, when the lens starts to turn yellow and slightly hazy. “This affects the patient by making his night vision not as good as it used to be,” explains Dr. Durrie, “and he will also usually need more light to read. It’s important to explain DLS in the patient’s terms—i.e., what they see, not what we see.”
The treatment options for stage II DLS are still in the elective category. “When they get into stage II, the corneal inlay option goes away,” says Dr. Durrie. “Blended vision/monovision is still an option but is only a solution for stage II—you need to explain that eventually they’ll need to have their lens replaced when DLS progresses. So, this leads to the more likely treatment discussion for individuals in their 50s and 60s: Is it maybe time to get the crystalline lens replaced with an intraocular lens by way of refractive lens exchange and, in doing so, prevent cataracts?”
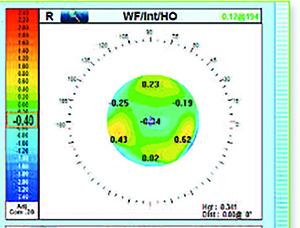 |
| The OPD-Scan III can show the physician the amount of aberrations caused by the lens vs. the amount caused by the cornea. (Image courtesy Nidek/Marco.) |
• Stage III. When the lens is defined as entering stage III, it simply means it has a cataract as defined by Medicare, which is usually acuity of 20/50 or worse with or without glare, or which involves visual impairment that severely hinders one’s daily activities.
Diagnostic Instrumentation
Instrument companies are also getting involved in promoting the concept of DLS, with devices that make it easier to analyze the crystalline lens.
One device that’s used by DLS proponents is the HD Analyzer (Visiometrics, Barcelona). “It was one of the first devices to popularize the idea of ocular scatter, or how much light is lost when rays travel through the optical system,” says Dr. Durrie. “It provides an optical scatter index and a simulated visual acuity reading. When diagnosing DLS, if I have a patient with an ocular scatter index of 0.5 or less, that means he has outstanding optics. His tear film, cornea, lens and vitreous have no ocular scatter. We absolutely know his lens is still clear and, if he’s in his 40s, has stage I. Patients with OSI between 0.5 and 1 are still considered normal and not who we’d be looking at for lens procedures, but we might have to look at them more closely and ask why their optics aren’t pristine. Once the OSI value gets above 1.0, there’s ocular scatter present, but it can be coming from the ocular surface, corneal problems such as keratoconus, et cetera. At that point you have to conduct more discussions and diagnoses to determine where the distortion is coming from.”
Another instrument some surgeons find useful is the iTrace (Tracey Technologies, Houston), which has a dysfunctional lens index function. The DLI will separate out the eye’s aberrations, showing the clinician the amount coming from the cornea and the amount from the lens. “The iTrace also has an index number that it will give you,” explains Dr. Piracha. “A 10 is a perfectly clear lens, and a zero is a dense cataract. Anything below 5.0 is significant for cataract and if the patient is at a 6.0 or 7.0, he’s getting close.” The Nidek/Marco OPD-Scan III can also tell the user how much of the aberration is internal (lens) vs. external (cornea). “You can also use the Oculus Pentacam to look at the lens and see how much of a density increase there is,” says Dr. Durrie. “So it’s not just one piece of equipment in DLS diagnosis, but it involves equipment some surgeons have in their offices already, or new equipment that the physician will add in order to have this DLS discussion with patients.”
Dr. Waring says the diagnostics help refractive-surgery decision-making in general. “Where the advanced diagnostics have been extremely useful has been in guiding the refractive surgeon in the critical aspects of making the decision between a cornea- or lens-based procedure,” he says. “So, in stage I, where the first dysfunctionality is experienced in terms of a loss of accommodation and where there’s still a relatively clear lens as determined by diagnostics and the clinical exam, patients would benefit from a corneal procedure. In stage II, in which the lens has become less clear and the patients are only presenting to get out of glasses and do not subjectively or objectively qualify for an insurance-based cataract procedure, these patients may benefit more from a lens-based procedure relative to a corneal-based procedure. Either way, patients are reassured that this is a normal, age-related and ubiquitous process.”
Controversies with DLS
There some surgeons who take issue with DLS, either with particular aspects of how it’s treated, such as removing a clear lens, or simply with the idea that the profession needs a new term at all.
Boulder, Colo., surgeon Mark Packer says that, though he has no problem with refractive lens exchange as an elective procedure, he thinks the rationale behind DLS cedes too much power to payors in terms of making diagnoses. “The issue I have with the promotion of dysfunctional lens syndrome is that its proponents are basically trying to create a diagnostic category for patients with visual acuity that’s too good for third-party payors to consider approving for cataract surgery,” he says. “Fundamentally, I believe that we, as physicians, shouldn’t allow Medicare or insurance companies to govern what we consider diagnostic categories. That’s the practice of medicine. I don’t like the idea that we’re making up diagnostic categories so they fit within the parameters that have been determined by payors. My primary motivation is: What does the patient want? What is the complaint? If it’s a refractive complaint, it’s very clear that it’s out of pocket, and that’s fine. But then there’s the person who gets the best pair of glasses I can find and still can’t drive well at night. That’s different. I cringe at this formulation of a diagnostic category that’s simply based on the premise: ‘The patient’s acuity is better than 20/40, so Medicare isn’t going to pay, but we want somebody to pay, so let’s call it dysfunctional lens syndrome and get more patients to pay for their own surgery.’
“I will say that the original idea behind dysfunctional lens syndrome was coming from a good place,” Dr. Packer adds. “There are people with functional problems who don’t meet the criteria for worse than 20/40 best-corrected vision necessary for cataract surgery to be reimbursed—so the question arose regarding what we were going to do for them. My answer would be, let’s not call it something new, but instead let’s call it what it is: It’s either a cataract or it’s serious optical aberrations, and there are already ICD codes for those. The treatment for those diagnoses is to replace the lens, giving the patient better vision. If someone has a functional problem and his best-corrected acuity is 20/20, that’s what he has health insurance for; that’s a medical problem and there are ways to document it.”
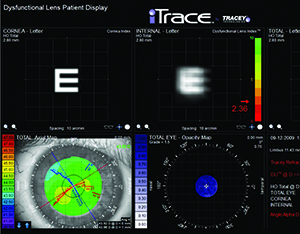 |
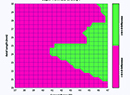
| The dysfunctional lens syndrome functions on the iTrace show how internal aberrations (i.e., from a patient’s crystalline lens) impact visual acuity by showing how much blur would occur with a standard letter E. (Image courtesy Tracey Technologies.) |
“In contrast sensitivity testing, for example,” explains Dr. Packer, “it’s pretty well-accepted that a 0.3 log-unit decline from the normative value of whatever instrument you’re using is significant. I usually go with the peak, which is usually 3 or 6 cycles/degree. The manufacturer can supply normative data of what the peak should be. The VF-14 questionnaire is another valid testing instrument. A score of 75 percent or below on the questionnaire indicates that the patient would benefit from cataract surgery. Basically, if you score above 75 percent, you’re already seeing well and there’s not a lot of room to improve, so postop satisfaction scores will drop. But at 75 percent and below, you’ll see a high rate of postop patient satisfaction from cataract surgery; in other words, people are saying, ‘This solved my problem.’ On the other hand, I’ve had patients say they were having a problem but score 95 percent on the VF-14—so there actually wasn’t a problem.”
For a patient with a complaint, even if Snellen acuity in normal lighting conditions doesn’t qualify him for cataract surgery, glare testing might. “Usually what’s done is the physician uses glare testing to show that it drops the patient’s acuity,” says Dr. Packer. “If a patient reads the chart at 20/20, but when you put on the brightness acuity tester he only reads 20/50, everyone would agree that’s significant. But what if it only drops to 20/30? In that case, you’d need some other documentation, such as contrast sensitivity with glare. That’s a much more sensitive indicator than high-contrast acuity. A patient may also have a so-called wavefront cataract, meaning there’s little in the way of lens opacity but there’s a whopping amount of aberrations in his lens and he’s got functional complaints stemming from it. For that, I’d say there’s a diagnostic code, ‘optical aberrations,’ and the treatment for it is lens extraction, and then I’d document why I determined that. Maybe that’s sort of a risky place to be for some surgeons, and some would rather say, ‘I know you have functional complaints, but your insurance isn’t going to pay for this, because they have particular visual acuity criteria.’ My answer to that would be, yes they have that criteria, but there are clauses within those rules that say some things that give you a little bit of latitude that you can work with. Document the patient’s functional complaint, such as not being able to drive despite the best pair of glasses, and then create a file documenting your findings. Perform the surgery and bill the third-party payor. If they don’t pay or they audit you, you’ve got the function on the VF-14, the CS testing, your wavefront analysis and your quote from the patient of his impairment.”
Dr. Waring, however, points out that DLS does make allowances for patients in stage III (insurance-based cataract) to get cataract surgery that’s reimbursed by a third party. “The clear distinction is that DLS patients aren’t coming in complaining of dysfunction that’s affected their daily activities in the classic sense such as not being able to drive at night,” he says. “All patients are screened for such problems as difficulty driving at night due to glare. They’re also not qualifying for cataract surgery objectively in terms of decreased contrast sensitivity or BAT less than state standards—though it’s important to note that the requirements for the diagnosis of cataract differ from state to state. If a patient in the cataract age range presents with a complaint about wearing glasses or bifocals and is requesting to get out of them, we’ll screen him for cataracts and, if he’d benefit from a cataract extraction, we’d give him that as an option.”
Dr. Piracha says that, though he agrees some patients have better than 20/50 acuity but still have visual issues, he has to stay within the laws of his state. “Even though I know the cataract’s the problem, and can show the patient that the cataract’s the problem, the other problem is that he’s still 20/30, and according to insurance he’s not bad enough yet to qualify for cataract surgery,” says Dr. Piracha. “I’m very conscious of this because payors like to audit practices and not pay for procedures, and you don’t want to get in trouble for fraud. I follow the CMS guidelines, which use best-corrected Snellen acuity, best-corrected near and glare testing at medium power on the BAT. Normally, if we have someone who’s 20/30 or 20/40 with a lot of complaints and a beginning cataract, I’d say, ‘We’ll see you back in six months.’ ”
Surgeons who use the DLS diagnosis say another reason some colleagues may be waiting on DLS is because refractive lens exchange is a very involved procedure to undertake. “You have to have the tools to ensure the best results,” says Dr. Durrie. “You need intraoperative aberrometry to pick the IOLs carefully, you need to be able to do excimer touch-ups. You have to be comfortable with all these things before suggesting the removal of a lens that doesn’t have a cataract. I think many surgeons are still trying to decide on which side of the fence they’re going to sit on DLS.” REVIEW
Dr. Durrie is on the advisory boards of Visiometrics and AcuFocus. Dr. Waring is a consultant to AcuFocus. Drs. Piracha and Packer have no financial interest in the products discussed.