“We can quickly get our patients to the best treatment regimen possible by honing our diagnostic skills with the many new point-of-service diagnostic tests available, allowing us to differentiate between the different forms of ocular surface inflammation and dry eye,” says John Sheppard, MD, professor of ophthalmology, microbiology, and molecular biology at Eastern Virginia Medical School and president of Virginia Eye Consultants in Norfolk.
Robert Latkany, MD, founder of the New York Eye and Ear Infirmary’s Dry Eye Clinic, agrees. “We need to not treat every patient with the same regimen. True ocular surface specialists need to think outside the box, because people have different reasons why they are dry. That’s why there are various approaches to treatment. I still go with the theory that I will try certain things on patients, one or two at a time. If they work, I continue. If they don’t, I stop them and move on to my next option,” he adds.
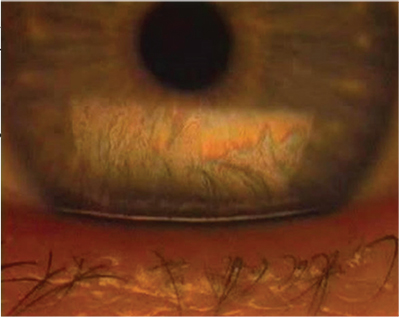 |
| Figure 1. Evaporative dry eye can greatly influence the oils of the tear film layer secreted by the meibomian glands. (All images: Karl Stonecipher, MD) |
There have been many advances in diagnostics in recent years. “The tear osmolarity test is one of the more commonly used point-of-care testing modalities available,” Dr. Latkany says. “There are varying opinions on it because of its extreme variability in measurement, but the bottom line is that it is available, and there is a reimbursable code for it. It is not very expensive, and it can offer some information for diagnosticians.”
Because the preoperative condition of the ocular surface can have an effect on postoperative outcomes, it is helpful to know if a patient has dryness before proceeding with cataract or refractive surgery. “Having these kinds of machines would help to prevent your possibly missing an asymptomatic dry-eye patient who may not say or know that she has dry eye,” Dr. Latkany says.
Rapid Pathogen Screening has the matrix metalloproteinase test, which is like a pregnancy test for detecting this inflammatory marker in the tear film. “It is nonspecific, but it can tell us if there is inflammation present in the tear film, and it can add some value in the potential diagnosis of certain ocular surface disease conditions. It is pretty simple to do, pretty inexpensive and sometimes reimbursable,” Dr. Latkany notes. Another diagnostic option is LipiView (TearScience), which can help detect irregularities on the corneal surface. “These machines are especially helpful to physicians who don’t know a lot about ocular surface disease,” he adds.
Treatment
The biggest news regarding the treatment of dry eye is the potential approval of lifitegrast (Shire), which is a small-molecule integrin antagonist that inhibits T-cell inflammation by blocking the binding of two key cellular surface proteins (LFA-1 and ICAM-1).
OPUS-2 was a multicenter, randomized, double-masked, placebo-controlled, parallel-arm study comparing lifitegrast (5% ophthalmic solution) to placebo administered twice daily for 84 days (12 weeks) in dry-eye patients with a history of active artificial tear use within 30 days prior to screening. The study included 718 patients at 31 U.S. sites and consisted of five visits over 98 days.1
Lifitegrast met one of the co-primary endpoints for the patient-reported symptom of improvement of dry eye compared with placebo, but it did not meet the second co-primary endpoint of the sign of inferior corneal staining. The secondary endpoints were descriptive only and were consistent with improvement in symptoms and lack of improvement in signs.
None of the patients in this study experienced serious treatment-emergent adverse events. The most commonly reported treatment-emergent adverse events associated with lifitegrast were dysgeusia (16.2 percent vs 0.3 percent for placebo), instillation site reaction (7 percent vs 1.1 percent for placebo), and reduced visual acuity (5 percent vs 6.4 percent for placebo).
“There will be a ruling from the FDA as early as July that will tell us the timetable to approval,” Dr. Sheppard says. “That approval would bring the prospect of two excellent prescription medications specifically indicated and approved by the FDA for dry eye. Having two heavily marketed agents available will really increase patient awareness. There are millions of dry-eye sufferers out there who don’t know they have dry eye and aren’t really under anyone’s care. With increased patient awareness, the hope is that these patients will actually seek out an eye doctor who will take care of that problem for them and avoid the potential morbidity that ensues from untreated or undertreated dry eye. Many times, these people are self-medicating with deleterious substances like vasoconstrictors or irritating generic eye drops that are laden with preservatives and actually do more harm than good.”
He notes that there are many excellent treatment options that have become available in the past decade. Prescription medications include Restasis and topical steroids, which provide excellent induction therapy for the initiation of dry-eye treatment, he explains. “In that regard, a company named Kala is looking for a specific induction indication for its enhanced pharmacokinetic preparation of loteprednol, utilizing a unique mucus-membrane-penetrating protein vehicle. That will provide, hopefully within the next several years, the first approved medication for induction of dry-eye pharmaceutical therapy,” Dr. Sheppard says.
“The treatments for blepharitis are becoming more exciting because we have the mainstays of lid scrubs, and we now have a very readily available topical hypochlorous acid,” he adds. “This is available in pure form as Avenova from NovaBay and also as Hypochlor from OCuSOFT. Applying this agent to the lids reduces bacterial counts and inflammation in a very effective manner.”
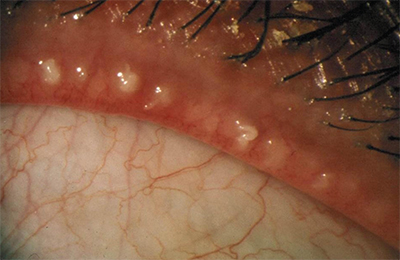 |
| Figure 2. Evaluation of the meibomian gland orifices. |
Some other exciting products are on the horizon, as well. “One product I’m working on with R-Tech Ueno is a recombinant topical human serum albumin, which is an outstanding, and apparently very safe, protein eye drop ready for Phase IIb trials,” Dr. Sheppard says. “Another exciting modality is Dextenza from Ocular Therapeutix, because their low-dose dexamethasone delivery system with a punctal plug is ideal for the dry-eye patient.”
Last year, Ocular Therapeutix released topline efficacy results from a Phase II clinical trial to evaluate the safety and efficacy of Dextenza (sustained release dexamethasone) 0.4 mg, intracanalicular depot for the treatment of allergic conjunctivitis.2 Dextenza is designed for extended drug release to the ocular surface for 30 days. The primary endpoint of treatment of ocular itching associated with allergic conjunctivitis was successfully achieved. There was a statistically significant difference in the mean scores between the Dextenza treatment group and the placebo group for ocular itching at all three time points measured on day seven post-insertion of the drug product.
Another new modality is the mimetogen secretagogue tavilermide (MIM-D3), which is now being developed by Allergan. In November, Allergan entered into an exclusive licensing agreement with Mimetogen Pharmaceuticals to develop and commercialize MIM-D3 (taviler-mide), a topical formulation of a novel small-molecule TrkA agonist for the treatment of dry-eye disease.
Top-line data have been released from the second clinical study with MIM-D3,3 and the trial demonstrated significant improvements in signs and symptoms with 1% MIM-D3 versus placebo, along with excellent safety, comfort and tolerability profiles.
The study, which included 403 patients, used Ora’s Controlled Adverse Environment chamber to measure dry-eye patients’ ability to withstand a stressful drying environment on the eye and patient diaries to measure the severity of dry-eye symptoms during the study. In this study, MIM-D3 was superior to placebo with regard to both central and total corneal fluorescein staining at week eight as measured by the Ora Calibra Scale. It also significantly improved common vision-related function symptoms of dry-eye disease as measured by the OSDK questionnaire. Additionally, the mean dry-eye scores for blurred vision, reading and watching TV were lower in the MIM-D3 group than in the placebo groups at week eight.
Study participants reported that MIM-D3 was comfortable and well-tolerated, and there were no unexpected or serious ocular adverse events. The most commonly reported ocular adverse events were reduced visual acuity (3 percent vs. 3 percent for placebo), instillation site pain (1 percent vs. 1.5 percent for placebo), and eye irritation (0 percent vs. 1.5 percent for placebo). All adverse ocular events were mild and transient.
“Another exciting development from Allergan is the now imminent availability of a preservative-free Restasis in a bottle because of the unique design of the filtration cap, which allows the patient to deliver repeated doses of totally preservative-free agent into the eye with a multi-dose container,” Dr. Sheppard says. “That will be greatly welcomed by patients who don’t like the tiny, single-dose units if they have arthritic hands and find the single-dose unit difficult to handle and squeeze. Finally, Allergan has acquired Oculeve, which introduces for the first time yet another mechanism of action for the treatment of the neurogenic component of dry eye. This device provides neural stimulation through the nasal mucous membranes to markedly increase production of tears through self-administration. I believe that Oculeve will meet an unmet need for yet another mechanism of action that was previously totally unaddressed. Hovione Scientia is developing a topical formulation of minocycline, overcoming legendary solubility and pharmacokinetic challenges for the dry-eye and meibomitis patient.”
He adds that ophthalmologists are increasingly using ProKera amniotic membranes from BioTissue to rapidly accelerate the healing of severe dry eye with an in-office procedure to apply the suture-free amniotic membrane. “For the most severe dry-eye patients, the PROSE (Prosthetic Ocular Surface Environment) contact lens, which is available in a dozen facilities around the country, can be custom-made for a very broad area that traps the tears and lubricates the eye, improves comfort and improves oxygenation and protection of the ocular surface,” he explains.
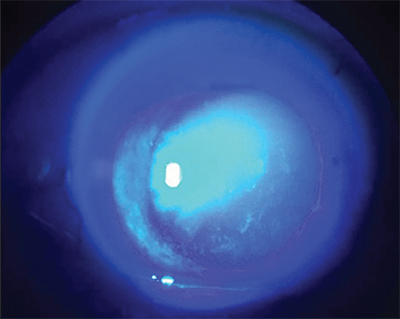 |
| Figure 3. Corneal fluorescein staining under blue light. |
Because so many patients have dry eye, it can be a daunting task to screen everyone. Karl Stonecipher, MD, empowers his technicians to screen for dry eye in the waiting room. “When patients walk in, there is a sign that says ‘Do you have dry eye?’ If they go to the effort to fill out the form, my technicians will use lissamine green or some other stain. If the screening is positive, it looks like the patient is dry, and she has staining, the next step may be tear osmolarity or InflammaDry (Rapid Pathogen Screening) or other options,” says Dr. Stonecipher, who is in private practice in Greensboro, N.C.
Technicians in his practice administer tests and questionnaires. “I really like the OSDI, because it is an app. I or one of the technicians can hand it to the patient while I’m doing EMR,” says Dr. Stonecipher. “The patient takes the test, and then it says mild, moderate, severe or normal. Then, the technician is empowered to go ahead and stain the patient. I like to diagnose the patient with a simple questionnaire while he is in the office waiting room before he is even seen by a technician. Then, the patient doesn’t have to see me and then go back to a technician to get repeat or additional testing. Basically, patients can be diagnosed and stained, and by the time they come to me, all I have to do is look at them and tell them their grade of dry eye. Next, I implement a treatment based on those diagnostics. For meibomian gland disease, I have enabled my technicians to be able to look at the eyelid margins and initiate appropriate diagnostics, such as LipiView.”
Dr. Stonecipher has developed a diagnostic and treatment center that is available to all doctors in the community. They can send patients over to get diagnostics done, and patients can get treatment. “I am all about teaching doctors how to diagnose dry-eye disease,” he says. “Once they can diagnose it, they need to be able to determine whether it is an aqueous-deficient dry eye, a meibomian gland disease or mixed mechanism, and then determine appropriate treatment. More and more people are taking the time and the effort to treat dry-eye disease, as opposed to referring the patient to a dry-eye specialist. It is economically profitable now, however far along the path you want to take it. By putting together a neutral diagnostic and treatment center, the doctors are referring their patients to these centers and then they get their patients back. It is the perfect co-management model.”
He empowers patients to learn more about their specific type of dry-eye disease, and he empowers technicians to learn as well, because he is unable to have these conversations with all of his patients. “I love talking to my patients, but, with the schedules I face today, that time is limited. If a lot of the education process has taken place before a patient comes to me, then we can focus on the patient,” he says.
Dr. Latkany has no financial interest in any of the products mentioned. Dr. Sheppard is a consultant for Alcon, Allergan, BioTissue, Kala, NovaBay, Rutech, Shire and TearScience. Dr. Stonecipher is a consultant for Alcon, Allergan and Shire.
1. Tauber J, Karpecki P, Latkany R, et al. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: Results of the Randomized Phase III OPUS-2 study. Ophthalmology 2015;122(12):2423-2431.
2. http://investors.ocutx.com/phoenix.zhtml?c=253650&p=irol-newsArticle&id=2100517.
3. http://www.firstwordpharma.com/node/1234316#axzz3FCBL SLlh




