The importance of detecting a relative afferent pupillary defect has been known for some time. Conditions that have been associated with RAPD include glaucoma, macular degeneration, ischemic retinal disease, retinal detachment, vitreous hemorrhage, optic neuritis and radiation damage. In the past, the swinging flashlight test was often performed in the clinic in an attempt to observe this sign, but the test is difficult to perform manually, not standardized and not easily quantifiable. Today, it’s less-often performed.
|
The idea of digitizing this test as a diagnostic tool has been successfully explored in the past, using the Procyon P3000 binocular pupillometer (Shwe Tin A, et al. IOVS 2009;50:ARVO E-Abstract 2471). Now, a recently available device called the RAPDx (Konan Medical, Irvine, Calif.) is expanding this possibility by allowing clinicians and researchers to test multiple variations of stimuli and timing.
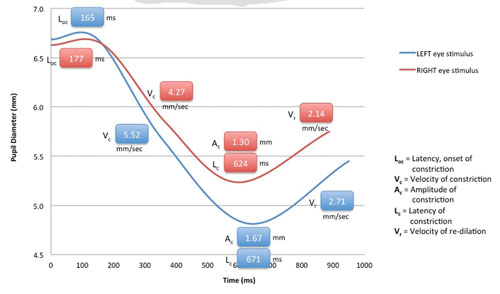
The RAPDx device records and quantifies how a subject’s pupils respond to various intensities, colors and patterns of light stimuli, including partial field stimuli (e.g., quadrants, macula-only, macula-sparing). While the patient is viewing binocularly, the device provides: monocular stimuli alternating between each eye (similar to the swinging flashlight test); automated eye tracking; simultaneous stimulation and recording; recording of responses in both eyes; auditory cues to simplify testing; and automated blink detection with automatic retesting for blink-affected responses. The instrument’s software rapidly analyzes pupil responses and provides numeric data. One form in which the data can be presented is the “RAPDx Signature,” a graph that displays details of the pupil responses over time, including constriction onset latency, velocity and amplitude, and recovery velocity. (See p. 18.) It can also overlay a normal, typical response curve for comparison. The company is developing normative databases.
Clinicians report that the device is easy to use; the standard testing function takes about three minutes. The test can be performed by a technician, and does not require any response from the subject.
RAPD and Glaucoma
Dolly S. Chang, MD, MPH, a PhD candidate at the Dana Center for Preventive Ophthalmology at Johns Hopkins University, is part of one of several teams investigating the use of the RAPDx device as a potential clinical tool, with a focus on discriminating patients with glaucoma from normals. (Her group is headed by principal investigator David S. Friedman, MD, MPH.) The group has worked with a prototype of the device—similar to the now commercially available version—since March 2011. They received no funding or support from Konan Medical other than the loan of the device.
|
“Glaucoma damage is often more severe in one eye,” notes Dr. Chang. “Not surprisingly, we found that patients with glaucoma had a greater asymmetry in pupil constriction than normal subjects. The difference in amplitude of pupil response was the strongest predictor, but the difference in time to constriction (latency) between the two eyes was also a strong predictor—although adding the latter predictor did not improve the algorithm’s diagnostic ability. The pupil response asymmetry also correlated with asymmetry in the visual field test or retinal nerve fiber layer thickness. These correlations might be explained by the loss of ganglion cells in eyes with glaucoma, resulting in smaller or delayed pupillary response.
“We also found that individual eyes with glaucomatous damage had smaller constriction and a delayed pupil response compared to normal eyes,” she continues. “Patients with milder disease often have relatively symmetric damage between the two eyes, resulting in fairly symmetric pupillary constriction, which diminishes the sensitivity of the between-eye comparison. That’s one reason we’re combining between-eye data and individual-eye data.”
Because glaucomatous damage is often asymmetric between the upper and lower visual fields, her group also assessed how pupils responded to stimulation of different parts of the retina. “The resulting values varied widely for both normal subjects and patients with glaucoma,” she says, “so this did not provide added information that might help us differentiate normals from those with glaucoma.”
Although the results are positive, Dr. Chang notes several limitations to their study. “For one thing, this is a select clinic-based study, and we’re optimizing our algorithms for maximum performance,” she says. “Real-world performance could be better or worse. Second, other eye conditions, such as severe cataract, macular degeneration or diabetic retinopathy also affect pupillary response to light, so further testing would be needed to make a diagnosis. Third, although this device compensates for blinking and incorporates eye tracking and other features, there are still some patients who cannot complete the test, or blink too frequently. Previous ocular surgery or an irregular iris shape can also limit accurate measurement, and there are other confounders that may affect pupil response such as age, medications and other comorbidities. Finally, our sample size is small.”
Only the Beginning
Dr. Chang notes that this device isn’t ideal as a glaucoma diagnostic tool—at least right now—because multiple conditions can trigger the kind of abnormalities the instrument picks up. “However, it still has the potential to be used as a screening tool for eye diseases in general,” she says. “People displaying an abnormal pupil response can be referred for further investigation. On the positive side, this test is non-invasive, involves minimal risk and doesn’t require a patient response, so it should be useful in situations in which a patient cannot successfully take a visual field test.
“We need further studies to assess the reproducibility of the test results, and longitudinal studies to assess whether the changes in pupil response over time are correlated with other visual tests,” she concludes. “That will help us determine the applicability of the test for long-term monitoring of glaucoma progression.” REVIEW