When faced with a patient who has ocular hypertension but no glaucomatous damage, deciding whether to treat or simply monitor the patient is always a judgment call. We have to consider the risks and benefits of each option. How likely is it that this patient will develop glaucoma and progress? Is that risk great enough to offset the inconvenience and cost of treatment?
These questions become a bit trickier when the patient is elderly.
How many more years is the patient likely to live? Can the patient manage drops? Will the patient be able to return for follow-up visits? Does the patient have other health issues?
 |
| Although it’s tempting to assume that elderly patients won’t live too many more years, many of these patients are vibrant, healthy and active. |
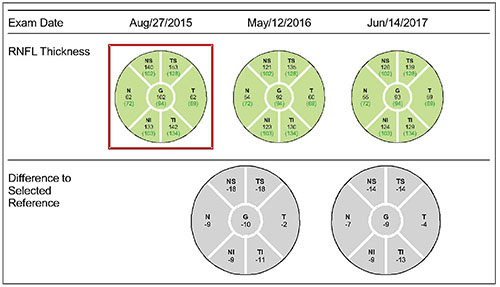
For example, consider an 86year-old male patient who has ocular hypertension; his pressure over the past two years has range between 27 and 31 mmHg OD, and 27 and 33 mmHg OS. His corneas are a bit thin—523 μm OD, 520 μm OS. He has a small cup-to-disc ratio of 0.2 bilaterally and his visual fields are normal. OCT reveals that his retinal nerve fiber layer has been thinning over time—a case of so-called “green disease”—but it’s still within the normal range. He’s pseudophakic with mild macular degeneration, but he’s generally in good health and doesn’t have vasculopathic risk factors such as high blood pressure, diabetes, hyperlipidemia, cardiovascular disease or history of stroke, all of which can predispose someone to a vein occlusion or anterior ischemic optic neuropathy.
Does this 86-year-old patient actually have early glaucoma? Is treatment really necessary? Here, I’d like to discuss some of the considerations that arise when deciding whether or not to treat a patient like this.
The Case for Not Treating
There are several reasonable arguments for monitoring this patient without treatment:
• The risk of developing early glaucoma in at least one eye over five years is low. Depending on how you calculate the risk, it’s between 10 and 15 percent. Those odds are very much in favor of no damage occurring over the patient’s expected lifespan.
As noted in a 2007 study of a model designed to predict the development of primary open-angle glaucoma in individuals with ocular hypertension, a predictive model should never replace clinical judgment.1 Other factors, such as a patient’s health, life expectancy and preferences must be considered. In our sample case, the patient is healthy and has no significant vasculopathic risk factors, so that would be an argument for not treating this patient and just watching.
 |
| Although it’s tempting to assume that elderly patients won’t live too many more years, many of these patients are vibrant, healthy and active. |
• A small amount of progression is unlikely to affect quality of life. The possibility that an 86-yearold patient might progress isn’t necessarily a reason to treat. Even if this patient does progress a little bit in the years ahead, it’s unlikely to affect his quality of life.
When treating glaucoma patients, I think we tend to get caught up in the idea that we don’t want anybody to progress. But preventing progression isn’t ultimately our goal; what we’re really trying to do is maintain quality of life. In that context, if a 96-year-old patient has a tiny bit of glaucoma, does it matter? If your patient has a little nasal step at age 86, should you be concerned? Of course, if that patient is a fast progress or and likely to develop an arcuate scotoma, yes, that matters. But a little nasal step probably won’t affect that person’s quality of life—and that’s what this is all about.
• It’s very easy, practical, affordable and effective to follow our patients with OCT. This is especially valuable when managing an older patient who may or may not be very good at doing visual field tests. So, you could leave this patient’s elevated IOPs untreated, check the IOPs at reasonable intervals and do an OCT once a year. If the OCT continues to change, then you can implement treatment.
Glaucoma specialist Cynthia Mattox, MD, has noted her own move in this direction. She has said that earlier in her career she treated for IOP levels, but today, with OCT to help with monitoring and the data from OHTS, she’s more comfortable following patients who have a low five-year risk of progression.
• Some patients in this age group might have difficulty taking a drop. If that’s the case, a prescription would probably be ineffective and a waste of the patient’s money.
• Some patients could find the cost to be a burden. In that situation, the patient is unlikely to use the drops even if you prescribe them.
The Case for Treating
Arguments in favor of treating this patient include:
• He has several risk factors for progression. One tool we’ve used to help guide our treatment is the data from the European Glaucoma Prevention Study and the Ocular Hypertension Treatment Study (OHTS), both of which looked at the risk of progression. This particular patient has several risk factors, including his age, his fairly high IOP and his thin cornea, so his five-year risk of progression in one eye is about 10 to 15 percent. (Of course, he also has a small cup-to-disc ratio, and normal Humphrey visual fields and pattern standard deviation—which is why there’s some debate about proceeding with treatment.)
 |
| * From Social Security Actuarial Life Table, https://www.ssa.gov/oact/STATS/table4c6.html. Accessed July 9, 2018. |
• This patient is very healthy and could live many more years. It’s entirely possible that this 86-year-old patient could live to be 100. Although his five-year risk of progression is low—around 15 percent—the longer he lives, the greater the risk.
Despite the lingering stereotype of elderly people as somewhat feeble individuals, many of the elderly patients I see are vigorous and in good health. Especially today, a person in his or her 80s and 90s can have a significant additional life expectancy. (In fact, I’ve observed that you don’t live to be 95 unless you’re a pretty healthy person.) With a younger patient it’s safe to assume the individual will be around for many more years, but it’s a lot more challenging to guess the life expectancy of an elderly patient.
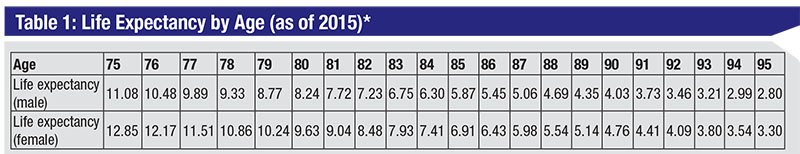
One approach to estimating your patient’s likely lifespan, and therefore the likelihood of developing glaucomatous damage, is to examine actuarial tables. These report the average length of time individuals in a given age group will live. (See sample table, above.) Based on this table, the average life expectancy for this patient is a bit more than five additional years. However, this is an average, and averages can’t predict an individual case. And of course, the longer he lives, the greater the likelihood he’ll experience progression and damage.
• Treatment with a PGA once a day is relatively low-cost and low-risk. Putting the patient on a PGA once a day is a very simple and reasonable treatment—assuming that the patient is able to put in a drop every day.
• We don’t know for sure that the patient will keep his follow-up appointment. I practice in a suburb at the very edge of Chicagoland, and a lot of my patients come in from the western farmlands of Illinois. It may take them an hour and a half to get to my office. In addition, the patient’s family situation could make it hard for the patient to come in regularly. If he doesn’t return for follow-up appointments, our ability to catch any developing problem will be compromised. Those kinds of issues would make me feel more inclined to treat.
• We’re concerned that the elevated IOP might increase the risk of an AION, CRVO or branch vein occlusion. Although there’s an association between these vascular events and elevated IOP, we don’t know for sure that lowering IOP decreases the risk of these types of vascular events. In the OHTS study, for example, the data indicated no statistical difference in the incidence of retinal vein occlusion in subjects who were treated in order to lower IOP compared to subjects who were not treated.2 Nevertheless, given the association between high IOP and these vascular issues, we tend to err on the side of wanting to lower the IOP, just to “play it safe.”
Even if lowering IOP hasn’t been shown to reduce the risk of a vascular event, clinicians may have reason to be cautious. If you let the IOP remain high and the patient does have a vascular event like an AION or branch vein occlusion later on—despite the lack of risk factors present when you examined the patient—you’ll have a tough time defending your choice to monitor rather than treat. For this reason, some would argue that treating a patient like this is a good idea simply because of the medicolegal risks posed by the association between high IOP and vasculopathic events.
• We’re afraid of missing somebody who might be a fast progressor. Treating the patient, in other words, is a way to hedge our bets that this patient might develop glaucoma more quickly than we expected.
Louis Cantor, MD, made the observation that we tend to overtreat glaucoma suspects and undertreat real glaucoma. I think that’s true. It’s easy to put people on drops, and we tend to do that whenever we’re not sure about whether to treat. Ironically, part of the motivation would seem to be to make ourselves feel better.
The Word on the Street
All of these arguments have some validity, but I was curious to see what ophthalmologists practicing in the real world would do in a situation like this.
To begin, I conducted an informal poll of several university employed and private practice glaucoma specialists to see whether or not they would choose to treat this elderly ocular hypertensive individual. There was no clear preference. Five colleagues said they’d monitor without treatment because the five-year risk of progression is fairly small. Seven of my colleagues said they would definitely treat with a PGA.
Three others said they might treat the patient with selective laser trabeculoplasty, especially if the patient had poor memory or dexterity or some other reason for having difficulty using drops. SLT is a great option if you have adherence concerns with an elderly patient, and it’s less expensive than a drop because it’s a one-time cost. However, SLT doesn’t always work, and some might argue that it’s a questionable idea because you’re doing a procedure in a “normal” eye that doesn’t have any damage yet.
I also polled the audience at one of my meeting presentations, and got a somewhat different response: The audience was overwhelmingly in favor of treating the patient with a PGA because of the high IOPs. I suspect the difference in responses may reflect a sampling error. The casual sampling of a group of colleagues at two academic meetings is likely to include glaucoma specialists who are highly data-driven, doctors who apply the OHTS and EGPS data to decision-making. In this instance, many of them recognized that the risk of progression was low and concluded that they wouldn’t recommend treating. However, I think many clinicians out in the field are pretty uncomfortable letting a patient’s pressure stay at 30 mmHg, regardless of other factors.
Getting the Patient On Board
What would I do? When faced with this situation, I’d let the patient help me decide whether or not to treat. In my experience, patients are usually smart and fairly sophisticated, and they usually have an opinion. I’d present the data from OHTS and explain it to the patient. I’d tell her that it wouldn’t be unreasonable to hold off on treating and simply monitor her condition, but I’d also tell her that high pressure is easy to treat.
In most cases, given the option, patients choose to go ahead with treatment—but not always. That’s important, because if the patient declines treatment he’s probably telling you that he won’t use the drops, even if you prescribe them. On the other hand, if the patient helps make the decision to treat, that helps to motivate the patient to use the drops consistently.
Ultimately, some would say that the best test of a decision is to ask whether you’d make the same decision if the patient were your own parent or child. My parents are 87 and 88 years old, and quite robust and healthy. Would I treat one of them if they had an intraocular pressure of 30, despite everything else appearing healthy? I probably would. REVIEW
Dr. Williams is a glaucoma consultant at Wheaton Eye Clinic in Wheaton, Ill., and past president of the American Academy of Ophthalmology. She is a consultant for Allergan and Aerie.
1. Ocular Hypertension Treatment Study Group; European Glaucoma Prevention Study Group, Gordon MO, Torri V, Miglior S, et al. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology 2007;114:1:10-9.
2. Barnett EM, Fantin A, Wilson BS, Kass MA, Gordon MO, for the Ocular Hypertension Treatment Study Group. The incidence of retinal vein occlusion in the ocular hypertension treatment study. Ophthalmology 2010;117:3:484–488.



