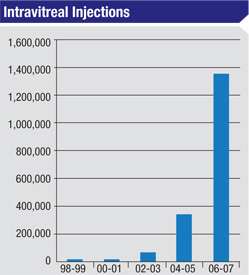
Intravitreal injection of anti-vascular endothelial growth factor, or VEGF, agents has become the standard of care for the treatment of a number of retinal diseases, including exudative macular degeneration; retinal vein occlusions; diabetic macular edema; proliferative diabetic retinopathy; and neovascular glaucoma. The most serious complication from intravitreal injection of anti-VEGF agents is endophthalmitis. Although rare, endophthalmitis can result in devastating loss of vision or loss of the eye.
|
Several groups have reported their experience regarding the incidence of endophthalmitis after intravitreal injection and found rates of endoph-thalmitis ranging between 1/1,300 to 1/10,000; however, these studies were limited by their retrospective nature and relatively small sample size.4-8 Recently, a large, single-center, consecutive case series with a prospective surveillance log found the incidence of clinically suspected endophthalmitis to be approximately 1/1,500.9 However, the incidence may indeed be lower than 1/1,500, as the culture-positive rate in the largest study to date was only 34 percent,9 in contrast to the 69 percent culture-positive rate in the Endophthalmitis Vitrectomy Study.10
There are several possible reasons for this lower culture-positive rate. All patients who were felt to have clinically suspected endophthalmitis after intravitreal injection underwent vitreous tap with injection of antibiotics, thus patients who may have had a sterile inflammatory reaction to either bevacizumab or ranibizumab may have been included in this series. Alternatively, patients with post-injection endophthalmitis often underwent in- office vitreous and/or anterior chamber tap only, while many patients in the EVS had a vitreous biopsy with a vitrectomy instrument. The larger vitreous sample may have increased the culture yield.
Presentation
Patients with endophthalmitis typically present with decreased vision and pain. In a recent series, all cases of intravitreal injection-related endophthalmitis presented with pain, decreased visual acuity and vitritis on average 3.4 days after injection. In addition, 18 of 23 cases (78 percent) had a hypopyon.9 However, the presence or absence of pain, vitritis, decreased vision and hypopyon did not distinguish between culture-positive and culture-negative cases, suggesting one should maintain a low threshold to treat presumed infectious endophthalmitis. Fortunately, most cases returned to baseline vision within several months, although a few had significant visual loss.
Antibiotic Issues
Clinicians have looked at a number of risk factors for the development of endophthalmitis following intravitreal anti-VEGF injection. Much attention has been given to topical antibiotic use before, during and after the injection. Studies have failed to show direct evidence that topical antibiotics given prior to the intravitreal injection reduce the risk of endophthalmitis. The best study on topical perioperative drops demonstrated that topical povidone 5% reduced the incidence of endophthalmitis occurring after cataract surgery; extrapolating this data, povidone should be used during the prep for intravitreal injections.11 The evidence concerning the efficacy of topical antibiotic use is conflicting. One study found that compared to topical antibiotic drops started three days prior to eye surgery, 5% povidone-iodine solution as part of the procedural prep resulted in a similar decrease in the number of bacterial colonies and species cultured from the conjunctiva; however, when used together they produced a synergistic effect and yielded the greatest reduction in bacterial colony counts.12
In contrast, more recent work suggests that although bacterial colony counts can be reduced by the use of topical antibiotic drops administered several days prior to the procedure, topical antibiotic drops did not appear to reduce colony counts significantly more than topical povidone as part of the prep alone.13 Another recent paper from the DRCR.net group found a low rate of endophthalmitis in a protocol that did not use topical antibiotics other than topical povidone as part of the procedure prep.14 Although the study size was small, this prospective data suggests that the benefit of topical antibiotic use is at best limited.
The manner in which topical povidone is used is also important. After administration of a topical anesthetic such as proparacaine, povidone should be placed so that it is in direct contact with the conjunctiva prior to administration of any viscous anesthetic gel (if used by the treating physician). If the topical viscous anesthetic is placed onto the conjunctiva before betadine use it acts as a barrier to sterilization.15 A recent clinical study found no cases of infectious endophthalmitis in a consecutive series of 4,690 patients receiving anti-VEGF agents using topical betadine immediately before and after instillation of 2% topical lidocaine gel anesthesia.16
The VEGF Inhibition Study In Ocular Neovascularization (VISION) trial suggested the risk of endophthalmitis could be modified by vigilance to an aseptic injection technique. In addition, use of a lid speculum was suggested as a prevention strategy.17 However, bladed lid speculum use, displacement of the conjunctiva, hemisphere of injection and type of anti-VEGF agent (i.e., bevacizumab vs. ranibizumab) did not affect risk in a large retrospective study.9
The most common organisms to cause endophthalmitis after cataract surgery are coagulase-negative Staphylococcus and Staphylococcus aureus, which make up approximately 75 percent of isolated bacteria.18 Most bacteria originate from the patient’s native flora.19 The EVS study found that one of the major predictors of visual outcome in patients who developed endophthalmitis was microbial etiology.10 Cases that were culture-positive for Staph epidermidis had better visual outcomes than cases positive for more virulent organisms such as Streptococcus or gram negative organisms.
The microbial spectrum seen in cases of endophthalmitis following cataract surgery differs from the microbial spectrum seen following intravitreal injection of anti-VEGF agents. Of note, several studies have shown an increased rate of streptococcal cases following intravitreal injections of anti-VEGF agents. In the EVS study, 9 percent of the culture-positive isolates were Streptococcus species,10 and another study found 8.2 percent of the culture-positive isolates following clear cornea cataract surgery were Streptococcus species.18 In contrast, a meta-analysis of endophthalmitis after intravitreal injection of anti-VEGF agents found 30.8 percent of culture-positive isolates to be Streptococcus species.20 The preponderance of Streptococcus endophthalmitis after intravitreal injection was confirmed by two other studies.9,21
This variation in the microbial spectrum has generated several hypotheses regarding the origin of the organisms. Adult salivary flora is made up of at least 41 percent Streptococcus species22,23 and aerosolization or droplet spread can serve as potential contaminates in the operative field.24-28
This method of transmission has been best studied for lumbar puncture. Although the incidence of meningitis after dural puncture is much lower than the incidence of endoph-thalmitis after intravitreal injection, there are several similarities between the procedures: Both involve insertion of a needle into a nutrient-rich body in a non-operating room setting, and both the spinal fluid and the vitreous are immunologically privileged sites.20 Because of the potentially fatal outcome associated with meningitis, risk factors for the development of post-spinal tap meningitis have been extensively studied, especially regarding use of a surgical mask. Two studies found that the absence of a surgical mask was associated with a significant increase in colonies per exposed plate.27,29 Other studies have shown that there is an increase in colony counts when not wearing a facemask and talking, as opposed to wearing a facemask or not talking.20,26
Another study concerning meningitis after dural puncture demonstrated an increased risk of bacterial spread during times of concurrent viral upper respiratory tract infection in a health-care provider.25 Furthermore, the authors found a surgical mask was effective in decreasing spread of bacteria during the study.25 Comparisons to intraarticular knee injection have also been made, and studies have shown that failure to wear a facemask resulted in increased infection with alpha-hemolytic Streptococcus organisms.30 Given the support these studies provide for mode of transmission of bacteria via aerosolization or droplet spread, it may be advisable to wear a facemask during the procedure or avoid talking, sneezing, or coughing in order to prevent transmission of upper respiratory tract organisms.9,20,31
Fortunately, the incidence of end-ophthalmitis after intravitreal injection is low, and most eyes regain lost vision over time. However, some patients have poor outcomes, partly due to organism virulence. Clinicians should maintain a low threshold for treatment of suspected endophthalmitis as pain, vitritis, decreased vision and hypopyon do not appear to differentiate between culture-positive and culture-negative cases.9 Povidone-iodine should continue to be the mainstay of endophthalmitis prevention, as peri-procedural use of topical antibiotics, use of lid speculum, hemisphere of injection, and use of bevacizumab versus ranibizumab do not appear to affect risk. Finally, work remains to be done to better reduce the incidence of endophthalmitis following an increasingly common procedure.
REVIEW
Dr. Theventhiran is a first-year resident in ophthalmology at Temple University Medical School. Dr. Shah practices at Ophthalmic Consultants of Boston. Dr. Garg practices at MidAtlantic Retina, is an associate professor of ophthalmology at Thomas Jefferson University and is on the Retina Service at Wills Eye Institute.
1. Taban M, Behrens A, Newcomb RL, et al. Acute endophthalmitis following cataract surgery: A systematic review of the literature. Arch Ophthalmol 2005;123:613-20.
2. Ramulu PY, Do DV, Corcoran KJ, Corcoran SL, Robin AL. Use of retinal procedures in medicare beneficiaries from 1997 to 2007. Arch Ophthalmol 2010;128:1335-40.
3. Heimann H. Intravitreal Injections: Techniques and Sequelae. In: Holz FG, Spaide RF, eds. Medical Retina: Springer Berlin Heidelberg; 2007:67-87.
4. Cavalcante LL, Cavalcante ML, Murray TG, et al. Intravitreal injection analysis at the Bascom Palmer Eye Institute: Evaluation of clinical indications for the treatment and incidence rates of endophthalmitis. Clin Ophthalmol 2010;4:519-24.
5. Fintak DR, Shah GK, Blinder KJ, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina 2008;28:1395-9.
6. Mason JO 3rd, White MF, Feist RM, et al. Incidence of acute onset endophthalmitis following intravitreal bevacizumab (Avastin) injection. Retina 2008;28:564-7.
7. Klein KS, Walsh MK, Hassan TS, et al. Endophthalmitis after anti-VEGF injections. Ophthalmology 2009;116:1225 e1.
8. Diago T, McCannel CA, Bakri SJ, Pulido JS, et al. Infectious endophthalmitis after intravitreal injection of antiangiogenic agents. Retina 2009;29:601-5.
9. Shah CP, Garg SJ, Vander JF, et al. Outcomes and Risk Factors Associated with Endophthalmitis after Intravitreal Injection of Anti-VEGF Agents. Ophthalmology 2011;Publication Pending.
10. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol 1995;113:1479-96.
11. Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology 1991;98:1769-75.
12. Isenberg SJ, Apt L, Yoshimori R, Khwarg S. Chemical preparation of the eye in ophthalmic surgery. IV. Comparison of povidone-iodine on the conjunctiva with a prophylactic antibiotic. Arch Ophthalmol 1985;103:1340-2.
13. Ta CN. Topical antibiotic prophylaxis in intraocular injections. Arch Ophthalmol 2007;125:972-4.
14. Bhavsar AR, Googe JM Jr., Stockdale CR, et al. Risk of endophthalmitis after intravitreal drug injection when topical antibiotics are not required: The diabetic retinopathy clinical research network laser-ranibizumab-triamcinolone clinical trials. Arch Ophthalmol 2009;127:1581-3.
15. Doshi RR, Leng T, Fung AE. Povidone-Iodine Before Lidocaine Gel Anesthesia Achieves Surface Antisepsis. Ophthalmic Surg Lasers Imaging. 2011 Jul-Aug;42(4):346-9.
16. Inman ZD, Anderson NG. Incidence of Endophthalmitis after Intravitreal Injection of Antivascular Endothelial Growth Factor Medications Using Topical Lidocaine Gel Anesthesia. Retina 2011;31(4):669-72.
17. VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N) Clinical Trial Group. D’Amico DJ, Masonson HN, Patel M, et al. Pegaptanib sodium for neovascular age-related macular degeneration: Two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology 2006;113:992-1001.
18. Lalwani GA, Flynn HW, Jr., Scott IU, et al. Acute-onset endophthalmitis after clear corneal cataract surgery (1996-2005). Clinical features, causative organisms, and visual acuity outcomes. Ophthalmology 2008;115:473-6.
19. Speaker MG, Milch FA, Shah MK, Eisner W, Kreiswirth BN. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology 1991;98:639-49; discussion 50.
20. McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina 2011 Apr;31(4):654-61.
21. Chen E, Cox J, Brown DM. Ten Years of Endophthalmitis at a Single Tertiary Retina Practice: Comparing Intravitreal Injection Cases Versus Post-Anterior Segment Surgery Cases. Presented at American Society of Retina Specialists (ASRS) Annual Meeting; September 1, 2010; Vancouver, Canada.
22. Gordon DF, Jong BB. Indigenous flora from human saliva. Appl Microbiol 1968;16:428-9.
23. McCarthy C, Snyder ML, Parker RB. The Indigenous Oral Flora of Man. I. The Newborn to the 1-Year-Old Infant. Arch Oral Biol 1965;10:61-70.
24. Veringa E, van Belkum A, Schellekens H. Iatrogenic meningitis by Streptococcus salivarius following lumbar puncture. J Hosp Infect 1995;29:316-8.
25. Sheretz RJ, Reagan DR, Hampton KD, et al. A cloud adult: The Staphylococcus aureus-virus interaction revisited. Ann Intern Med 1996;124:539-47.
26. O’Kelly SW, Marsh D. Face masks and spinal anaesthesia. Br J Anaesth 1993;70:239.
27. McLure HA, Talboys CA, Yentis SM, Azadian BS. Surgical face masks and downward dispersal of bacteria. Anaesthesia 1998;53:624-6.
28. Trautmann M, Lepper PM, Schmitz FJ. Three cases of bacterial meningitis after spinal and epidural anesthesia. Eur J Clin Microbiol Infect Dis 2002;21:43-5.
29. Philips BJ, Fergusson S, Armstrong P, Anderson FM, Wildsmith JA. Surgical face masks are effective in reducing bacterial contamination caused by dispersal from the upper airway. Br J Anaesth 1992;69:407-8.
30. Reeves KD, Horvat RT. Aerosolized alpha-hemolytic Strep-tococcus as a cause of knee sepsis after intra-articular injection: Predisposing factors. Am J Phys Med Rehabil 2010;89:77-82.
31. Wen JC, McCannel CA, Mochon AB, Garner OB. Bacterial dispersal associated with speech in the setting of intravitreous injections. Arch Ophthalmol 2011;129:1551-4.