One of the toughest parts of telling a patient that he or she is
developing age-related macular degeneration is informing the patient
that, currently, little can be done to help. Of course, recent studies
of nutritional supplements have offered some hope, and those studies
continue to proliferate—albeit with sometimes contradictory results.
(Among the recent entries: Data from the Carotenoids in Age- Related Eye
Disease study suggests that high concentrations of vitamin D may
protect against early macular degeneration in women younger than 75
years of age, although it may increase the odds of developing the
disease in women older than 75.1 And a recent multi-year study of 38,022
women without any diagnosis of macular degeneration at the outset found
evidence that regular consumption of docosahexaenoic acid,
eicosapentaenoic acid and fish was associated with a significantly
decreased risk of incident macular degeneration during the 10-year
follow-up period.2) Unfortunately, these studies are largely focused on
prevention. Furthermore, the best outcomes are not seen in every
subject.
Now, however, the prognosis for dry AMD may be about to improve; numerous ideas for treatment are showing promise in clinical and preclinical trials. They cover a wide range of approaches, including managing enzymes that influence RNA behavior; slowing the visual cycle to reduce damage; increasing blood flow in the eye to restore normal function; new laser treatments; stem cells; high-tech implants to deliver drugs long-term; an antioxidant-rich spice; neuroprotection; and various drugs that reduce inflammation.
Enzymes and RNA
One promising new approach to addressing dry AMD has come from the work of Jayakrishna Ambati, MD, professor of physiology and ophthalmology, and his team of researchers at the University of Kentucky. They’ve discovered a molecular process that appears to lead to geographic atrophy, and they’re developing ways to interfere with that process.
Dr. Ambati says his group’s approach to research has been to examine
diseased post-mortem eyes to discover how they compare to healthy eyes.
“As described in our published paper on this topic,3 we found that in
human donor eyes with geographic atrophy there’s a dramatic reduction in
the abundance of an enzyme called Dicer,” he says. “Dicer regulates
many important processes, including the creation of micro-RNA, and we
found that reducing Dicer in various cell cultures and mouse eyes caused
the kind of changes that we see with geographic atrophy. [The
published study showed that this result did not occur when other similar
enzymes were reduced.] Through a series of experiments we found that a
deficiency of Dicer leads to an accumulation of an RNA substance called
Alu transcripts, and we were able to show that Alu transcripts are
directly toxic to RPE cells. In fact, taking this toxic material from
human eyes and putting it into mouse systems causes the disease to occur
in those systems.”
Dr. Ambati explains that this suggested three possible approaches to blocking the process. “In our paper we discussed two approaches: increasing Dicer abundance and blocking Alu RNA,” he notes. “Since then, we’ve confirmed the changes in Dicer and Alu RNA in more human eyes, and also created a non-human primate model of the disease. In addition, we’ve developed a third approach to blocking the process: We’ve now identified how the Alu RNA is signaling and causing the death of RPE cells, and we’ve found small molecules that can block that downstream signaling.”
Dr. Ambati says each of the three possible approaches has advantages and disadvantages. “Increasing Dicer levels would probably have to be done using something like viral gene therapy,” he says. “Unfortunately, that process is long and arduous, and the regulatory guidelines are not entirely clear and delineated. Also, Dicer is a protein that has many important functions, and it might be risky trying to get it to levels that are exactly right. So we’re not very fond of that approach. Blocking Alu RNA using anti-sense molecules is interesting, but there are a lot of problems with anti-sense technology. The third approach—using small molecules to block the downstream effects of Alu RNA—is something we’re becoming more fond of, because it’s amenable to a small molecule delivery strategy and may be compatible with drug delivery systems that others are developing.”
Dr. Ambati says his group is in conversation with pharmaceutical companies about licensing this technology, and they’re preparing for a clinical trial. “We’ve established a database of patients, identified a reading center and have a variety of clinical trial centers both here and abroad,” he notes. “People are very eager to collaborate with us. We plan to start Phase I trials in the next few months; ultimately we’ll have to seek FDA approval to market the agent we use in the trials. If everything goes well, we should have the Phase I trial completed by the end of 2012.”
Nonthermal Nanolaser
Dr. Ambati explains that this suggested three possible approaches to blocking the process. “In our paper we discussed two approaches: increasing Dicer abundance and blocking Alu RNA,” he notes. “Since then, we’ve confirmed the changes in Dicer and Alu RNA in more human eyes, and also created a non-human primate model of the disease. In addition, we’ve developed a third approach to blocking the process: We’ve now identified how the Alu RNA is signaling and causing the death of RPE cells, and we’ve found small molecules that can block that downstream signaling.”
Dr. Ambati says each of the three possible approaches has advantages and disadvantages. “Increasing Dicer levels would probably have to be done using something like viral gene therapy,” he says. “Unfortunately, that process is long and arduous, and the regulatory guidelines are not entirely clear and delineated. Also, Dicer is a protein that has many important functions, and it might be risky trying to get it to levels that are exactly right. So we’re not very fond of that approach. Blocking Alu RNA using anti-sense molecules is interesting, but there are a lot of problems with anti-sense technology. The third approach—using small molecules to block the downstream effects of Alu RNA—is something we’re becoming more fond of, because it’s amenable to a small molecule delivery strategy and may be compatible with drug delivery systems that others are developing.”
Dr. Ambati says his group is in conversation with pharmaceutical companies about licensing this technology, and they’re preparing for a clinical trial. “We’ve established a database of patients, identified a reading center and have a variety of clinical trial centers both here and abroad,” he notes. “People are very eager to collaborate with us. We plan to start Phase I trials in the next few months; ultimately we’ll have to seek FDA approval to market the agent we use in the trials. If everything goes well, we should have the Phase I trial completed by the end of 2012.”
Nonthermal Nanolaser
Another promising new approach to treating early macular degeneration involves treating the retina with a nanosecond laser with the power reduced to sub-thermal levels. Twelve-month data from a non-randomized, prospective clinical pilot study involving 50 high-risk patients suggests that the treatment reduces drusen and has a beneficial effect on visual function.
The treatment, called Retinal Rejuvenation Therapy, or 2RT, was developed by Ellex (Adelaide, Australia). The trial was partly funded by the Victorian government and conducted by the Centre for Eye research Australia, University of Melbourne, at the Royal Victorian Eye and Ear Hospital. Laser treatment in the highest-risk eye of each participant consisted of either 12 single pulses of 3-ns duration, placed in a “clock face” pattern around the mid-macula, or two linear patterns of six shots following the superior and inferior arcades. Followup is being performed at three, six and 12 months, with completion of the 12-month follow-up targeted for the end of December 2011.
Interim 12-month results for 24 patients presented at the 2011 meeting of the Association for Research in Vision and Ophthalmology demonstrated that approximately two-thirds of patients experienced an improvement in visual function and/or drusen reduction in the treated eye. Interestingly, the majority of patients also noted an improvement in the fellow, untreated eye, and the treated areas of the retina did not correlate with the location of visual function improvement. Instead, visual function improved predominately in the regions of greatest dysfunction. (There was no decrease in function in the treated areas, and no adverse effects were noted.)
“This treatment is aimed at trying to reduce or slow down early disease—where there are usually no symptoms but the retina shows signs of drusen and pigment change—or even reverse the progression to either dry or wet,” notes the study’s principal investigator, Professor Robyn Guymer, head of the Macular Research Unit at the Centre for Eye Research Australia.
“The aim at Ellex was to develop a laser that could deliver beneficial effects to the RPE/Bruch’s membrane interface without any of the harmful effects potentially caused by thermal lasers. To accomplish that, the 2RT laser treatment uses 500 to 1,000 times less energy per laser spot than previous thermal lasers. So far, we’re not sure what the mechanism of action is, but it is clearly systemic, because a beneficial effect is also seen in the fellow eye.
“Next,” she adds, “we hope to do a masked, randomized study with large enough numbers over a long enough time period to show that this treatment really does result in less progression to late AMD.”
Stem Cells
As with many other physical ailments, researchers are hopeful that stem cells may be able to help manage macular degeneration. Several universities and health-care organizations in the United States and England are investigating this possibility.
One company, Advanced Cell Technology, received FDA approval in January to begin trials using human embryonic cells for this purpose. The company coaxes the stem cells into becoming RPE cells; the idea is that the transplanted cells will replace damaged RPE cells before all RPE function is lost. The initial study will test the safety of the modified cells and how well the subjects tolerate them over a 12-month period.(Concurrent with the macular degeneration study, the company will be running a similar study with patients who have Stargardt’s disease.) ACT has been developing the technology for the past decade, and some animal studies have suggested that it is effective. ACT recently announced the enrollment of the first patient in the Phase I/II prospective, open-label trial, at the Jules Stein Eye Institute at UCLA. Eleven more patients will be enrolled by mid-summer. The subjects will be divided into groups to test different doses of the cells.
Earlier this year, researchers at Georgetown University Medical Center also published a study documenting their success at creating retinal cells from stem cells.4 They demonstrated that RPE cells generated from human induced pluripotent stem cells can exhibit ion transport, membrane potential, polarized VEGF secretion and gene expression profile similar to those of a normal eye’s RPE cells. However, the lead author on the study, Nady Golestaneh, PhD, has noted that the group is not yet ready to attempt transplantation; the altered cells display some problems that appear to be related to the use of viruses to reprogram the cells.
Research is also under way at Columbia University Medical Center in New York, and the School of Science at Indiana University-Purdue University in Indianapolis.
|
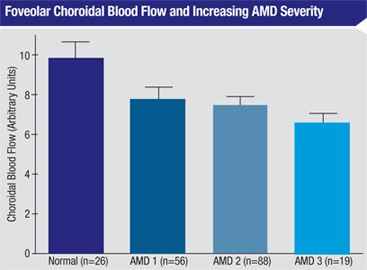
MacuCLEAR, a clinical-stage specialty pharmaceutical company started at Texas A&M several years ago by George Chiou, PhD (best known for leading the discovery of timolol, the pioneering treatment for glaucoma), is currently developing MC-1101, a proprietary eye drop intended to stop dry stage macular degeneration from progressing to the wet stage by enhancing blood flow in the back of the eye. The active ingredient of MC- 1101 has been previously approved by the FDA as an oral antihypertensive drug, so its safety and toxicity profile is well-established.
Studies conducted by the company have demonstrated that despite being topically applied, MC-1101 reaches the retina, relaxes the epithelial lining of the vasculature, dilates choroidal blood vessels by stimulating nitric oxide production and increases blood flow. The argument for using this approach to treat dry macular degeneration centers around the idea that the fundamental difference between dry AMD and wet AMD is the integrity of Bruch’s membrane. Restoring unrestricted blood flow should normalize the removal of debris and toxins, thus controlling inflammation and oxidation of tissues of the retina and preventing neovascularization, in turn preventing the rupture of Bruch’s membrane.
“MC-1101 is based on a vascular model developed by Dr. Chiou,” explains Phil Ralston Jr., CEO of MacuCLEAR. “Dr. Chiou realized that the retina requires more oxygen than any other tissue in the body, and many ophthalmic luminaries have reported on the coincidence of low choroidal blood flow and macular degeneration.5,6 We know that with aging, you get an atherosclerotic reduction of blood flow in the choroid, leading to a dysfunction in the ability to remove the waste occurring in the back of the eye. We also know that when the body is deprived of oxygen, it builds collateral blood vessels.
“Many other approaches [to treating dry AMD] try to remove one factor from the disease process, but the process continues,” he notes. “We’re trying to restore the mechanism that effectively prevents disease in the two-thirds of us who don’t develop it—the mechanism that manages all of those other factors.”
“Today, with the FDA’s guidance, we’re preparing to start a series of Phase III studies designed to show the efficacy of our drug, measuring visual function with agreed-upon endpoints,” Mr. Ralston adds. “If the first, smaller study produces good results, we’ll do a second trial with more patients. We hope to start the first study early in the fourth quarter of this year.” MC-1101 has been granted 505(b)(2) status with Fast Track designation from the FDA.
Slowing the Visual Cycle
Abnormal
accumulation of lipofuscin and retinol-derived toxins, including A2E,
have been implicated in the development and progression of geographic
atrophy. For that reason, reduction of circulating retinol is theorized
to be a potential treatment for the development of GA, and fenretinide, a
synthetic derivative of vitamin A, has been shown to reduce levels of
serum retinol binding protein (RBP), which in turn reduces delivery of
retinol to the eye.
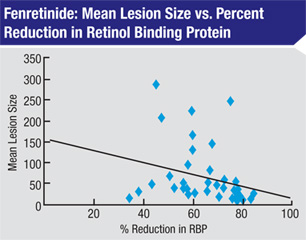
A recently completed two-year, randomized, multicenter study measured the effect of fenretinide on 246 patients with geographic atrophy; 153 completed the full two years of treatment. Fifty-nine received placebo, 47 received 100 mg of the drug each day, and 47 received 300 mg per day.
A recently completed two-year, randomized, multicenter study measured the effect of fenretinide on 246 patients with geographic atrophy; 153 completed the full two years of treatment. Fifty-nine received placebo, 47 received 100 mg of the drug each day, and 47 received 300 mg per day.
|
- In subjects who experienced at least a 60-percent drop in serum RBP, the median lesion growth rate over the two years was 1.28 mm2/year; in the placebo group, the rate was 2.03 mm2/year, a significant difference.
- In subjects who experienced at least a 60-percent drop in serum RBP, atrophic lesion size remained stable between months 12 and 24, resulting in a loss of six letters; those in the placebo group lost 11 letters during the same period.
- In the 300-mg group, median lesion growth was 30 percent from baseline; in the placebo group it was 50 percent.
- Patients in both fenretinide groups had half the risk of progressing to wet macular degeneration at two years, compared to placebo.
“One of the potential targets for treating dry macular degeneration is modifi cation of the visual cycle,” he explains. (Other drugs being tested using this approach include Acucela’s ACU-4429, an oral drug currently starting a Phase III trial. It appears to reduce accumulation of toxic fluorophores and lipofuscin, preventing photoreceptor and RPE cell loss.) “There’s a lot of evidence that the damage caused by geographic atrophy is damage to the RPE cells from a buildup of toxic byproducts from the visual cycle—i.e., the recycling of photopigments. These byproducts are supposed to be processed and carried out of the cells, but in many patients they build up and apparently poison the cells. Unfortunately, the only way we know of to slow the accumulation of these byproducts is to slow down the normal visual process.”
Dr. Slakter notes that blood levels were also tested during the trial. “Animal studies had already indicated that a physiologic effect required achieving at least a 60-percent reduction in RBP,” he says. “In this trial, all but one of the subjects on the original formulation—the group that did so well—had a greater than 60-percent reduction in RBP. Furthermore, there was a correlation between the amount of RBP suppression and the change in atrophy rate and vision preservation.
“One of the things this indicates is that you need to suppress RBP below a certain level to have an effect,” he notes. “Thus, it offers a biomarker; a blood test at one month might reveal whether or not a patient is going to respond to this treatment. It also might allow you to titrate the drug. As long as you achieve suppression of 60 percent, you’ll probably get the beneficial effect, and you might be able to reduce the dose.”
Dr. Slakter acknowledges that night vision is likely to be affected by the drug. “You’re trading off long-term preservation of central vision for some degree of night-vision difficulty,” he says. “However, less than 10 percent of patients in the trial had significant night-vision problems.”
In terms of preventing neovascularization, Dr. Slakter says he was surprised by that finding. “The rate was significantly lower in both fenretinide groups,” he says. “Apparently, fenretinide also acts through a different pathway to suppress VEGF. The threshold for that function may be lower, so you might see VEGF suppression with even less drug.”
Encapsulated Cell Therapy
Another novel approach to treating dry macular degeneration now being pursued at multiple centers, including the Shiley Eye Center at the University of California, involves long-term delivery of ciliary neurotrophic factor to the retina via encapsulated cell therapy.
|
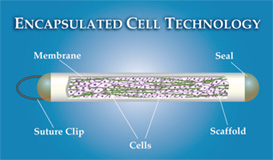
Encapsulated cell therapy refers to Implanting a capsule—a proprietary
technology developed by Neurotech Pharmaceuticals in Lincoln, R.I.,
called NT-501—into the back of the eye. The capsule, roughly the size
of a grain of rice, contains genetically engineered cells that
continuously produce CNTF for up to 24 months, according to the company.
The capsule allows the CNTF to diffuse into nearby tissues while
allowing oxygen and nutrients into the capsule to nourish the
drug-producing cells; at the same time, it protects the cells inside
from antibodies and the immune system.Implanting the capsule is done as
an outpatient procedure that takes 15 to 20 minutes, and the capsule can
be removed later if necessary.
In a Phase II, multicenter, double- masked, sham-controlled trial, 27 geographic atrophy patients received the implant while another 24 patients underwent a sham surgery or were implanted with a lower dose. The primary endpoint was change in BCVA at 12 months. The high-dose eyes showed a significantly reduced amount of change in macular volume at 12 months compared to the sham group; all but one patient in the high-dose group (96.3 percent) lost fewer than three lines of vision, compared to 75 percent of the sham group. The high-dose group also showed increased retinal thickness that correlated with vision stabilization. Furthermore, an analysis of a subgroup of subjects who began the trial with vision of at least 20/63 or better found that 100 percent of the high-dose group (n=10) maintained stable visual acuity, whereas only 55.6 percent of the low-dose and sham-treated subjects in this category did (n=9). However, no increase in BCVA (the primary endpoint of the study) was observed.
In this study, as well as others using this technology to treat other conditions, no adverse events have been associated with the surgical procedure or the implant. Neurotech has received fast-track designation for NT-501 for the treatment of visual loss associated with dry macular degeneration.
In a Phase II, multicenter, double- masked, sham-controlled trial, 27 geographic atrophy patients received the implant while another 24 patients underwent a sham surgery or were implanted with a lower dose. The primary endpoint was change in BCVA at 12 months. The high-dose eyes showed a significantly reduced amount of change in macular volume at 12 months compared to the sham group; all but one patient in the high-dose group (96.3 percent) lost fewer than three lines of vision, compared to 75 percent of the sham group. The high-dose group also showed increased retinal thickness that correlated with vision stabilization. Furthermore, an analysis of a subgroup of subjects who began the trial with vision of at least 20/63 or better found that 100 percent of the high-dose group (n=10) maintained stable visual acuity, whereas only 55.6 percent of the low-dose and sham-treated subjects in this category did (n=9). However, no increase in BCVA (the primary endpoint of the study) was observed.
In this study, as well as others using this technology to treat other conditions, no adverse events have been associated with the surgical procedure or the implant. Neurotech has received fast-track designation for NT-501 for the treatment of visual loss associated with dry macular degeneration.
Adding a Bit of Spice
A recent study conducted at multiple universities
in Italy7 found that short-term supplementation with saffron, a spice
containing the antioxidant carotenoids crocin and crocetin, improved
some measures of vision in patients with early macular degeneration.
Twenty-five patients received 20 mg/day of oral saffron or placebo for
three months, followed by switching to the alternative for three more
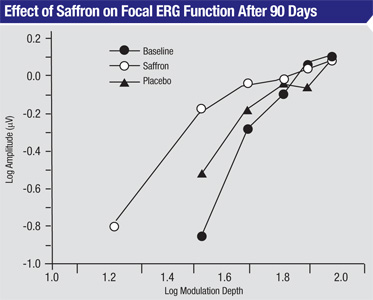
months. After saffron supplementation, focal electroretinograms
increased in amplitude and showed decreased thresholds, compared to
baseline or placebo (p<0.01). Clinical exams performed after 90 days
of supplementation also found that saffron subjects’ visual acuity
improved from 0.70 at baseline to 0.80; placebo subjects’ visual acuity
only improved to 0.72. The difference was significant (p<0.01).
A follow-up study of 29 early AMD patients was conducted to see whether visual improvement resulting from saffron supplementation would last out to one year. (Falsini B, et al. IOVS 2011;52:ARVO E-Abstract 127) After three months of supplementation, mean visual acuity improved by two Snellen lines compared to baseline values (0.75 to 0.9, p<0.01); mean focal ERG sensitivity improved by 0. 3 log units compared to baseline values (p<0.01). These changes remained stable over the follow-up period. A longer-term study is now being planned.
“We have preliminary evidence that as a consequence of saffron neuroprotection, the function of a pool of damaged but still viable cone photoreceptors in AMD eyes can be restored by saffron dietary supplementation,” says Benedetto Falsini, MD, associate professor of ophthalmology at the Department of Ophthalmology, Universita’ Cattolica, Policlinico A. Gemelli, in Rome. “The idea of using saffron supplementation to combat early macular degeneration was inspired by Prof. Silva Bisti’s experimental work on murine models of oxidative damage. That work showed a highly significant effect of saffron on both structure and function of the retina exposed to oxidative damage. We believe this effect is due to the effects of crocin and crocetin, saffron constituents and powerful antioxidants.”
Regarding the safety of saffron supplementation, Dr. Falsini says the study indicates that the doses employed in the study are safe. “It’s definitely something doctors can offer patients,” he says. “However, doctors need to remember that saffron is delicate and easy to manipulate. We use selected saffron previously tested in animal models, and the pills are prepared for us by a specialized company.”
Amyloid Suppression
|
A follow-up study of 29 early AMD patients was conducted to see whether visual improvement resulting from saffron supplementation would last out to one year. (Falsini B, et al. IOVS 2011;52:ARVO E-Abstract 127) After three months of supplementation, mean visual acuity improved by two Snellen lines compared to baseline values (0.75 to 0.9, p<0.01); mean focal ERG sensitivity improved by 0. 3 log units compared to baseline values (p<0.01). These changes remained stable over the follow-up period. A longer-term study is now being planned.
“We have preliminary evidence that as a consequence of saffron neuroprotection, the function of a pool of damaged but still viable cone photoreceptors in AMD eyes can be restored by saffron dietary supplementation,” says Benedetto Falsini, MD, associate professor of ophthalmology at the Department of Ophthalmology, Universita’ Cattolica, Policlinico A. Gemelli, in Rome. “The idea of using saffron supplementation to combat early macular degeneration was inspired by Prof. Silva Bisti’s experimental work on murine models of oxidative damage. That work showed a highly significant effect of saffron on both structure and function of the retina exposed to oxidative damage. We believe this effect is due to the effects of crocin and crocetin, saffron constituents and powerful antioxidants.”
Regarding the safety of saffron supplementation, Dr. Falsini says the study indicates that the doses employed in the study are safe. “It’s definitely something doctors can offer patients,” he says. “However, doctors need to remember that saffron is delicate and easy to manipulate. We use selected saffron previously tested in animal models, and the pills are prepared for us by a specialized company.”
Amyloid Suppression
At least two drugs being tested are designed to reduce the presence of amyloids, a component of drusen.
Copaxone, or glatiramer acetate, is a drug that’s been FDA-approved for treatment of multiple sclerosis; it suppresses T-cells and down-regulates infl ammatory cytokines. A Phase II/ III trial of the drug is currently underway at the New York Eye & Ear Infirmary. “Researchers in Israel discovered that copaxone has the ability to reduce drusen formation,” explains David Boyer, MD, in clinical practice with Retina-Vitreous Associates Medical Group in Los Angeles and a clinical professor of ophthalmology at the University of Southern California Keck School of Medicine.
“Pfizer also has a systemic anti-amyloid drug called RN6G that binds amyloid beta,” he adds. “It’s currently being used to treat Alzheimer’s disease, and they’re looking at the possibility of using it as a long-term treatment for reducing drusen.”
Neuroprotection
Several drugs being tested are taking this approach. Dr. Boyer is a participant in an ongoing trial of Tandospirone, aka AL-83098 (Alcon), a serotonin 1A receptor agonist delivered as a topical eye drop. “Animal studies have shown this drug to be neuroprotective,” says Dr. Boyer. “It reduces the waste products that are found at the edge of geographic atrophy.
Several drugs being tested are taking this approach. Dr. Boyer is a participant in an ongoing trial of Tandospirone, aka AL-83098 (Alcon), a serotonin 1A receptor agonist delivered as a topical eye drop. “Animal studies have shown this drug to be neuroprotective,” says Dr. Boyer. “It reduces the waste products that are found at the edge of geographic atrophy.
The current trial is a large Phase II trial designed to see whether the
drug can reduce the growth of geographic atrophy. All patients have
completed one year, and 18-month results— the pre-specified
endpoint—should be available soon.” Allergan’s brimonidine is also being
tested as a resource against dry macular degeneration.
“Brimonidine has been shown to be neuroprotective in a variety of different insults,” notes Dr. Boyer. “For this particular purpose the drug is injected intravitreally using the Ozurdex platform [currently used to deliver dexamethasone]. This releases the drug gradually over an extended time period.”
Other drugs under investigation as neuroprotective agents include trimetazidine, an anti-ischemic agent, the subject of a multicenter, randomized, double-blind, placebo-controlled, phase III study involving 1,100 patients in Belgium, France and Spain; and Alprostadil, a prostaglandin known to increase blood flow, currently in a Phase III trial in Europe.
Reducing Inflammation
“Brimonidine has been shown to be neuroprotective in a variety of different insults,” notes Dr. Boyer. “For this particular purpose the drug is injected intravitreally using the Ozurdex platform [currently used to deliver dexamethasone]. This releases the drug gradually over an extended time period.”
Other drugs under investigation as neuroprotective agents include trimetazidine, an anti-ischemic agent, the subject of a multicenter, randomized, double-blind, placebo-controlled, phase III study involving 1,100 patients in Belgium, France and Spain; and Alprostadil, a prostaglandin known to increase blood flow, currently in a Phase III trial in Europe.
Reducing Inflammation
Reducing inflammation is also seen as a promising avenue to pursue. Options being investigated include:
- Fluocinolone acetate. “Alimera Sciences is conducting a small study involving the Medidur implant,” says Dr. Boyer. “They’re investigating the possibility of using the steroid/device as a long-acting means of reducing the inflammatory component and hopefully triggering regression or a lack of progression of geographic atrophy.”
- Rapamycin (sirolimus). Once seen as a potential treatment for wet macular degeneration, sirolimus is now under investigation in an NIH-funded trial to determine its efficacy as a treatment for patients with bilateral geographic atrophy. Sirolimus inhibits the production, signaling and activity of many inflammatory factors relevant to the development of GA. The drug is being given in the form of multiple subconjunctival injections.
Dr. Boyer notes that there are many unanswered questions regarding the complement system approach. “We don’t know whether we need to use this type of drug systemically or locally, or how long we have to use it, and it’s not clear in many cases whether we should be using the drug for wet or dry disease,” he says. “Furthermore, these drugs inhibit different parts of the immune cascade. It’s not clear which is the best way to go with this.”
Help Is on the Way
None of the treatments on this list is currently ready for prime time.
But with so many different promising options under investigation, the
odds are good that you’ll soon have something to offer besides
comforting words and nutritional supplements. And that’s good news for
both you and your patients. REVIEW
1. Millen AE, Voland R, Sondel SA, Parekh N, Horst RL, Wallace RB, Hageman GS, Chappell R, Blodi BA, Klein ML, Gehrs KM, Sarto GE, Mares JA; CAREDS Study Group. Vitamin D status and early agerelated macular degeneration in postmenopausal women. Arch Ophthalmol 2011;129:4:481-489.
2. Christen WG, Schaumberg DA, Glynn RJ, Buring JE. Dietary omega-3 fatty acid and fi sh intake and incident age-related macular degeneration in women. Arch Ophthalmol. 2011; March 14 [Epub ahead of print].
3. Kaneko H, Dridi S, et al. DICER1 defi cit induces Alu RNA toxicity in age-related macular degeneration. Nature 2011;17:471:7338:325-30.
4. Kokkinaki M, Sahibzada N, Golestaneh N. Human Induced Pluripotent Stem-Derived Retinal Pigment Epithelium (RPE) Cells Exhibit Ion Transport, Membrane Potential, Polarized Vascular Endothelial Growth Factor Secretion, and Gene Expression Pattern Similar to Native RPE. Stem Cells 2011;29:825–835.
5. Ehrlich R, Harris A, et al. Age-related macular degeneration and the aging eye. Clin Interv Aging 2008;3:3:473–482.
6. Grunwald JE, Metelitsina TI, et al. Reduced Foveolar Choroidal Blood Flow in Eyes with Increasing AMD Severity. IOVS 2005;46:1033-1038.
7. Falsini B, Piccardi M, et al. Infl uence of Saffron Supplementation on Retinal Flicker Sensitivity in Early Age-Related Macular Degeneration. IOVS 2010;51:6118-24.