Usually, when researchers and engineers improve the speed of a device, its accuracy suffers. Conversely, if they work to make something laser-accurate, the process slows down. However, researchers from France say they've developed a rapid version of the polymerase chain reaction test that not only is more sensitive when it comes to identifying organisms responsible for endophthalmitis, it does it in 90 minutes instead of the hours or even days it takes to get results from cultures. Here's a look at their work, as well as the pros and cons of the new test.
Catching the Organisms
A PCR test involves extracting a minute sample and using a process called enzymatic replication to cause the sample organism's DNA, if present, to replicate multiple times to aid identification. Unlike a culture, the PCR needs to be equipped with a copy of the organism's DNA ahead of time in order for the test to begin replication of the same DNA in the sample. However, cultures have demonstrated a couple of weaknesses against endophthalmitis. "The biggest problem is when you look at any study of endophthalmitis, you'll get 25 to 30 percent rate of culture-negative endophthalmitis," says Nick Mamalis, MD, director of the ophthalmic pathology lab at the
Fast Real-time PCR
To overcome the disadvantages of cultures, Pablo Goldschmidt, PhD, of the Laboratoire du Centre National d'Ophtalmologie des Quinze in
The f-real-t PCR was highly sensitive, detecting 0.01 colony forming units of bacteria per microliter with no confounding cross-reactivity with fungi. It correlated 100 percent with the culture-positive results. The samples from non-infective cases tested negative. It even caught organisms that culture missed; 60 percent of the endophthalmitis samples tested culture-positive, but 90 percent were positive on f-real-t PCR. What's more, while it took several hours to a couple of days for the cultures to return an identification, the f-real-t PCR was complete in 90 minutes.
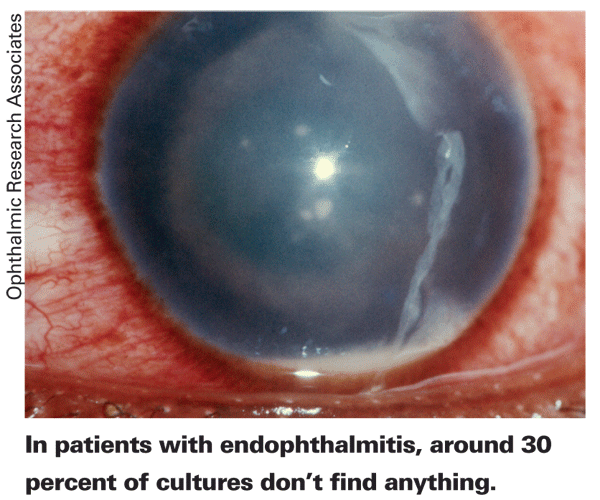
"It's a very simplified PCR method that gives a lot of information," says Dr. Goldschmidt. "Instead of waiting for a culture, or having nothing grow on a culture because the patient has already administered an antibiotic or the sample is too small, with the PCR you don't care if the bacteria grow or not because, whether they're dead or alive, you can still see them because you're looking for DNA."
Ashley Behrens, MD, a cornea and external disease specialist from the Wilmer Eye Institute, also feels that the PCR's sensitivity could be helpful in confounding cases such as toxic anterior segment syndrome, which resemble infection. "I had a case about six months ago in which the patient's eye behaved like a TASS case, but, after a few days, there was actually growth on the culture plate," Dr. Behrens recalls. "Then, there were more evident signs of endophthalmitis. But, in the beginning, I was wondering what to do—steroids or antibiotics? The two courses of therapy, are, of course, completely different. So, the greatest advantage of this PCR test is if we can get some sort of report that tells us, 'Yes, this sample has x, y and z bacteria in the aqueous or the vitreous,' then we can start thinking about treating an infection right away. Or, if it's negative, we could start thinking about TASS and a more conservative treatment."
Dr. Goldschmidt says one of the reasons the test is so quick is that it's keyed to endophthalmitis. "For bacteria, no one could do a PCR previously because researchers were attempting to detect 500 or 600 different strains," he says. "But, in ophthalmology, you just need to decide between five or six antibiotics for treatment. You don't need to do the whole battery of PCR testing. If it's Staphylococci, then you use vancomycin, for example. The point is to make it easy for ophthalmologists and not give them too much information, but rather give them just the information they need to decide on a treatment."
Christopher Ta, MD, of
Probably the biggest disadvantage, at least at this stage, is that PCR testing isn't widely available, and is often found only at academic centers or research hospitals. This lack of availability limits the number of clinicians who can actually use the test. Dr. Mamalis is hopeful that this situation will change if such testing is ultimately commercialized. "First, this will have to be done on an increased number of actual patients in order to confirm that it's a technique that's going to be better than the culture method we're doing right now," he says. "The other thing is the availability of this technique. This is something that's going to have to be done in more laboratories. At the moment, if you're a community ophthalmologist you don't have access to a laboratory that can perform PCR.
So this technique will need to be something that can readily be done in a smaller lab. But, like other technologies, as it becomes more widespread, hopefully the costs will go down and more labs will get experience in doing it, and it might be something that can be done in community hospitals."
1. Goldschmidt P, Degorge S, Benallaoua D, et al. New test for the diagnosis of bacterial endophthalmitis. Br J Ophthalmol. Published on-line Feb 10, 2009.