Cyclosporine has a relatively long history in ophthalmology. Originally, topical cyclosporine was used to prevent corneal transplant rejection. In the late 1980s and early 1990s, it was mixed in a 1 or 2 percent oil base, either peanut or corn oil, and was shown to be effective for that purpose.
More recently it has been mixed in a 0.5% artificial tear vehicle, which has also been shown to be effective in preventing graft rejection.
Unfortunately, because of the vehicles of oils and artificial tears, the high concentration of cyclosporine needed to obtain clinical efficacy, as well as issues with toxicity, pain, and irritation, cyclosporine A never became very popular, except in the hands of corneal specialists. Despite these drawbacks, several authors reported stellar clinical effects with topical cyclosporine. For most ophthalmologists, however, the difficulties in these proprietary concoctions of cyclosporine outweighed the benefits.
With the December 2002 Food and Drug Administration approval of Restasis (cyclosporine 0.05%, Allergan), the earlier drawbacks have been addressed. As longtime researchers of this product, we have devised a number of uses for Restasis in addition to its FDA indication, and will review them in this article.
Background
Cyclosporine A is the most important selective T-cell immunosuppressive agent available today. Topical cyclosporine's high clinical efficacy and superb safety profile make this the medication of choice for all ocular disease in which T-lymphocytes play a causative role. As cyclosporine is an immunomodulator and not an immunosuppressive, it offers advantages over traditional anti-inflammatories, such as corticosteroids:
• It inhibits T-lymphocytes, but does not inhibit the phagocytic system, and has no effect on viral replication.
• It does not increase intraocular pressure, as corticosteroids do.
• It does not decrease wound healing.
• It does not cause cataracts, and it significantly improves tear function.
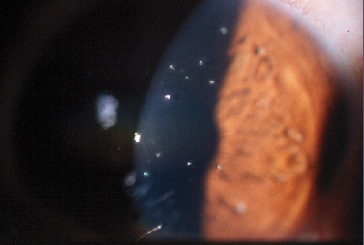 |
| Until now, patients with Thygeson's keratitis faced long-term steroid use. Restasis (cyclosporin 0.05%) may provide relief. |
The primary FDA-approved indication for Restasis is treating the immune-mediated dry eye. In the clinical trials, cyclosporine A was shown to significantly improve tear function by decreasing inflammation of lacrimal gland tissue, permitting patients to produce their own physiologically normal tears.
The Restasis vehicle, which is similar to that of Allergan's Refresh Endura, combines cyclosporine 0.05% in a lipid vehicle, which is extremely efficacious and well-tolerated. This lipid vehicle enables delivery of the drug at a much lower concentration, as shown by tear flurophotometry studies, and allows for an increased contact time of cyclosporine in the tear film of three to four hours, resulting in an increased absorption by the ocular surface.
We have shown that the Restasis medication of 0.05% essentially delivers slightly more cyclosporine to the ocular tissue than 1% and 2% cyclosporine in peanut oil, and at the same time, is much better-tolerated. The Restasis 0.05% is approximately half as efficacious as cyclosporine A 0.5% in a tear vehicle. These findings suggest that Restasis can be used successfully in preventing corneal transplant rejection.
Enhanced Transplant Care
We have three distinct indications for which we use cyclosporine A in our clinical practice.
We immediately start patients who have high-risk keratoplasties with neovascularization or who have suffered previous rejections on corticosteroids, such as Pred Forte, four times daily. We then provide additional prevention of rejection by adding Restasis four times daily. We taper both medications, but maintain the patients on both corticosteroids and cyclosporine A indefinitely if they are aphakic or pseudophakic. When the patient is phakic, we discontinue the corticosteroids after suture removal but maintain the patient on Restasis twice a day indefinitely.
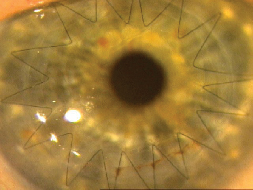 |
| Avoiding corticosteroids by substitution or adjunctive use of Restasis can also prevent steroid-induced hypertension. |
The second scenario involves patients who are at low risk for corneal transplant rejection, but in whom we want to facilitate wound healing. In these cases, such as keratoconic patients, we place the patient on Pred Forte four times daily, and Restasis two times daily. We then taper the steroids over approximately six weeks, while maintaining the patient on Restasis, usually two drops per day for three months, and then one drop daily. With this treatment, wound healing is expedited, and sutures can be removed at an earlier time frame.
The final application for cyclosporine A in the setting of corneal transplantation is for patients who are corticosteroid-responders and have developed steroid-related glaucoma. Glaucoma is seen in upwards of 70 percent of patients who have had previous keratoplasties, and corticosteroid-induced ocular hypertension occurs in 92 percent of patients with pre-existing glaucoma when treated with topical corticosteroids. We have shown that substitution of cyclosporine A for corticosteroids can produce a significant decrease in intraocular pressure in patients post-keratoplasty with corticosteroid-induced ocular hypertension.1,2 By substituting cyclosporine A for a corticosteroid on a 1:1 basis, we achieved a mean decrease in IOP of 8.2 mmHg in two groups totaling more than 75 patients.
Anti-fungal Activity
Cyclosporine A has also been shown to possess significant anti-fungal activity and is a very efficacious treatment for patients who have fungal infections in corneal transplants. Cyclosporine A can prevent allograft rejection, and coupled with its concomitant anti-fungal effect, makes it an important alternative to steroids in the management of a corneal transplant after mycotic infections.3,4
At this year's ARVO meeting, we presented a double-masked placebo controlled study on the use of topical Restasis in the treatment of posterior blepharitis (meibomian gland dysfunction). This study showed a trend toward improvement in all symptoms related to this condition and a statistically significant reduction in meibomian gland inclusions, telangiectasia, and corneal staining with the use of Restasis twice daily for three months.5
Cyclosporine A has been used for a long period of time in the treatment of peripheral non-infectious corneal ulceration, including rheumatoid corneal ulceration, Mooren's corneal ulceration, and psoriatic corneal ulcers.
Additional Uses
We have also used cyclosporine A in the treatment of Thygeson's keratitis.6 Thygeson's is a chronic inflammatory condition that results in a corneal epitheliopathy similar to epidemic keratoconjunctivitis, but in a white, quiet eye. Often these patients only respond to corticosteroids, which may be required for decades. We have found that Restasis is highly effective in managing these patients and they can be controlled with as little as one or two drops a week.7
 |
| Cyclosporine A may also have a role in preventing graft rejection. |
Superior limbic keratoconjunctivitis is often a difficult management problem. Many of these patients require long-term maintenance with topical corticosteroids. The associations of this condition with thyroid autoimmune disease and keratoconjunctivitis sicca led us to use topical cyclosporine A in its treatment. Our positive results are reported in the July 2003 Ophthalmology. Basically, the use of topical cyclosporine A obviates the need for topical corticosteroids in this condition.
One of the most important uses of topical cyclosporine A is in the management of vision-threatening allergic eye disease. These diseases include vernal keratoconjunctivitis,8,9 eczema, and atopic keratoconjunctivitis,10,11 all of which can cause corneal neovascularization into the visual axis. VKC can cause a shield ulcer, which can be vision-threatening. Generally these patients are managed with mast cell stabilizers, and when they do not respond to the medication, they are placed on topical corticosteroids, often for years at a time, with all the concomitant associated steroid-related side effects.
These diseases are generally bilateral inflammatory disorders that affect children and young adults, and they are associated with significant morbidity. In all of these diseases, topical cyclosporine has been shown to be highly effective in controlling the disease process.
Obviously there are multiple indications for Restasis in the management of T- cell-mediated ocular surface diseases. As physician comfort with cyclosporine A increases, new indications for its use will be elucidated over the next several years, with the understanding that any T-cell-mediated disease may benefit from topical cyclosporine A.
Cyclosporine A offers a unique combination of high therapeutic efficacy, specific T-cell modulation without affecting other inflammatory pathways, and most important an extremely low risk of ocular or systemic side-effects.
Supported in part by a grant from the Lions Club International Foundation, Oakbrook, Illinois, and an unrestricted grant from Allergan Pharmaceuticals.
Drs. Donnenfeld and Perry are in the Department of Ophthalmology, Nassau University Medical Center, East Meadow, N.Y. and in private practice. Contact Dr. Donnenfeld at Ophthalmic Consultants of Long Island, Ryan Medical Arts Building, Suite 402, 2000 North Village Ave., Rockville Centre, N.Y. 11570. E-mail: eddoph@aol.com.
1. Perry HD, Donnenfeld ED, Kanellopoulos AJ, Grossman GA. Topical Cyclosporine A in the management of post-keratoplasty glaucoma. Cornea 1997;16:284-8.
2. Perry HD, Donnenfeld ED, Acheampong A, et al. Topical Cyclosporine A in the management of post-keratoplasty glaucoma and corticosteroid-induced ocular hypertension (CIOH) and the penetration of topical 0.5% Cyclosporine A into the cornea and anterior chamber. CLAO J 1998;24:159-65.
3. Bell NP, Karp CL, Alfonso EC, et al. Effect of Methylprednisolone and Cyclosporine A on fungal growth in vitro. Cornea 1999;18:306-13.
4. Perry HD, Doshi SJ, Donnenfeld ED, Bai GS. Topical Cyclosporine A in the management of therapeutic keratoplasty for mycotic keratitis. Cornea 2002;21:161-3.
5. Perry HD, Doshi SJ, Donnenfeld ED. Topical Cyclosporine A in the treatment of posterior blepharitis. ARVO 2003.
6. Forstot L, Perry HD, Donnenfeld ED. Treatment of Thygeson's keratitis with topical Cyclosporine A. American Academy of Ophthalmology 1996.
7. Manvikar S, Figueiredo FC. Thygeson's superficial punctate keratitis: topical Cyclosporine A drops used in patients resistant to topical steroids. ARVO 2003.
8. Pucci N, Novembre E, Cianferoni A, et al. Efficacy and safety of Cyclosporine eyedrops in vernal keratoconjunctivitis. Ann Allergy Asthma Immunol 2002;89:298-303.
9. Gupta V, Sahu PK. Topical Cyclosporine A in the management of vernal keratoconjunctivitis. Eye 2001;15:39-41.
10. Hingorani M, Calder VL, Buckley RJ, Lightman S. The immunomodulatory effect of topical Cyclosporine A in atopic keratoconjunctivitis. Invest Ophthalmol Vis Sci 1999;40:392-9.
11. Hingorani M, Moodaley L, Calder VL, Buckley RJ, Lightman S. A randomized placebo-controlled trial of topical Cyclosporine A in steroid-dependent atopic keratoconjunctivitis. Ophthalmology 1998;105:1715-2.