Gary C. Brown, MD, MBA; Melissa M. Brown, MD, RN, MN, MBA, Philadelphia
Health-care costs have risen at twice the rate of general inflation over the past four decades.1 A potential dramatic upswing in health costs is likely to be further exacerbated by the aging of our population, a demanding baby-boomer sector, increased high-technology advances and continued rise in pharmaceutical prices.1-4 Thus, factoring health-care costs into the therapeutic equation is becoming a necessity, especially in view of newer expensive modalities such as the use of ranibizumab for the treatment of neovascular age-related macular degeneration.5
What then should be the priorities for vitreoretinal interventions? And at what cost? The answers can be found using value-based medicine. And although we think of vitreoretinal interventions as a subset of ophthalmic interventions, the answers to the questions are most relevant when compared more broadly with interventions across all of health care, especially since this is how insurers and others who allocate health-care resources rationalize their prioritization decisions.
Value-Based Medicine
Value-based medicine is the practice of medicine based upon the value (improvement in length-of-life and/or quality-of-life) conferred by health-care interventions.3,4 In doing so, value-based medicine uses the best evidence-based data to integrate value with costs using a health-care economic instrument called cost-utility analysis.
• Value-Based Medicine Principle 1. Use the highest level of evidence-based data, preferably randomized clinical trials and/or meta-analyses, in value-based medicine analyses.
In regard to value gain, length-of-life gain can often be culled from the literature, but the improvement in quality-of-life is more difficult to ascertain. It can, however, be quantified using utility analysis.3,4 Utility analysis objectively measures the quality-of-life associated with any health state, ophthalmic or otherwise. By convention, utility values range from anchors of 1.0 (normal health permanently) to 0.0 (death). For most ophthalmic interventions, 20/20 vision in each eye permanently is used as the upper, or 1.0, anchor.
• Value-Based Medicine Principle 2. Value-based medicine, cost-utility analyses should use standardized inputs (time trade-off utilities, patient utility respondents, direct medical costs and the Medicare Fee Schedule) and standardized outcomes (value gain in percent, QALY gain and $/QALY) to allow the comparability of all analyses across all of medicine.
We prefer the time trade-off variant of utility analysis, in which a patient is asked two questions: 1) How long do you expect to live? and 2) How much of that time, if any, would you be willing to theoretically trade in return for 20/20 vision in each eye permanently? In essence, time of life is traded for improvement in quality-of-life for the remaining time. The utility is then calculated by subtracting the proportion of time traded from 1.0. For example, the utility associated with 20/50 vision in the better seeing eye is 0.77,6 meaning the average person with eye disease and this level of vision would trade 23 percent of their remaining time of life in return for normal vision permanently. If an intervention improves the vision in the better seeing eye to 20/25, the corresponding utility increases to 0.87, a gain in utility of 0.10, or alternatively a 13 percent (0.10/0.77) gain in quality-of-life. Utilities are sometimes referred to as patient preferences, since they depend upon patient decisions about whether they prefer to trade time of life for a better health state, or prefer to trade no time and remain in the same health state.
Utilities used in a value-based medicine analysis should be derived from patients with the health state of interest, since they best know the quality-of-life associated with their condition. When ophthalmologists were asked to estimate the utility associated with different levels of macular degeneration, they had draconian underestimations ranging from 96 percent to 750 percent.7
Value gain. The total value gain conferred by an intervention is measured in quality-adjusted life-years (QALYs) gained.1,4 The QALY gain = (improvement in utility) x (duration of benefit in years). For example, if the 0.10 improvement in utility with visual improvement from 20/50 to 20/25 lasts for 15 years, the total gain is (0.87 – 0.77) x 15 = 1.5 QALYs.
People accrue, or gain, value as they go through life. If they lose vision to 20/50 in the better seeing eye and remain at that level for 20 years, they accrue 0.77 x 20 = 15.4 QALYs during that time. If an intervention improves their vision to 20/25 permanently for 20 years, they accrue 0.87 x 20 = 17.6 QALYs during the same period of time. Comparing the QALY accrual with treatment and without treatment over the duration of benefit, the total value gain is therefore 17.6 QALYs – 15.4 QALYs = 2.2 QALYs. 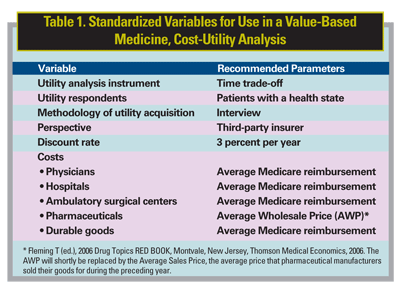
Ophthalmic interventions typically do not confer an improvement in length-of-life, but if the improvement in vision from 20/50 (utility of 0.77) to 20/25 (utility of 0.87) happened to increase the length-of-life by two years, an additional (0.87 x 2 =) 1.76 QALYs would be accrued for a total of 3.96 QALYs more accrued with treatment than without treatment.
While the total value gain conferred by an intervention could be calculated for a person of any age, a reference case analysis, or case of the average person with the disease of interest, is usually performed. This obviates the problem of discrimination against a group of patients due to age.4
In some instances the comparative value gain conferred by two different interventions is very obvious, but in others that is not the case; hence, a value-based medicine analysis becomes very relevant. For example, what confers more value for the treatment of subfoveal choroidal neovascularization? The improvement in mean long-term vision from 20/500 to 20/320 with laser photocoagulation, the improvement in mean long-term vision from 20/320+2 to 20/160+2 with photodynamic therapy with verteporfin (PDT), or the improvement from 20/200+1 to 20/126-1 in eyes treated with pegaptanib?8 Further, how do the adverse events and the incidence of adverse events associated with each of the respective interventions factor into the therapeutic equation?
• Value-Based Medicine Principle 3. Every patient should want, and should deserve, the intervention for his condition that confers the greatest value.
Costs. Once the value gain in QALYs is calculated for an intervention, the incremental costs, or costs associated with that treatment can be integrated. The costs used in value-based medicine analyses are direct medical costs (physicians fee, hospital fee, drug fees, etc.). Use of direct medical costs alone is referred to as the third-party insurer cost perspective. A societal cost perspective includes all costs (direct, caregiver, disability, etc.) and would theoretically be the best, but there is no agreement upon which costs to include and what should be the cost basis for each.
• Value-Based Medicine Principle 4. The costs associated with an intervention become relevant only when the value conferred by comparator interventions is similar, in which instance the less expensive intervention becomes the preferred intervention.
Cost-utility. Once in the form of $/QALY, or dollars expended per QALY, the $/QALY is known as the cost-utility, or the cost-utility ratio,4 although some authors also refer to it as a cost-effectiveness ratio.9 As an example, if the cost of the intervention that confers 1.76 QALYs is $17,600, the $/QALY = $17,600/1.76 QALYs = $10,000. An analysis that compares treatment to no treatment is referred to as an average cost-utility analysis, while an analysis that compares one treatment to another treatment is an incremental cost-utility analysis.
Rather than refer to interventions as cost-utilitarian, they are referred to as cost-effective or not cost-effective. The standards are soft, but interventions costing less than $50,000/QALY are considered to be very cost-effective, while those costing more than $100,000/QALY are not considered cost-effective.4 It is likely, as more interventions are studied with value-based medicine principles, that they will be categorized according to deviations from the mean and/or median.4
In the
Vitreoretinal Interventions
The value gain, cost and cost-utility associated with a number of vitreoretinal and other interventions are shown in Table 2. The underlying evidence-based data for each fulfill Value-Based Medicine Principle 1.
The cost for each is calculated over the years of interventional benefit for the reference case. For example, the reference case timeline is 12 years for neovascular macular degeneration therapies,10,11,15 12 years for cataract surgery,17,18 23 years for hypertension therapy and 19 years for statin therapy. Each of the analyses herein is comparable since all adhere to Value-Based Medicine Principle 2.
Cataract surgery in the initial eye confers the greatest ophthalmic interventional value, while intravitreal ranibizumab (Lucentis) confers the greatest value for the treatment of neovascular macular degeneration. Of note is the fact that each of the interventions for the treatment of neovascular macular degeneration confers greater value than the treatment of hyperlipidemia with statins, the latter being among the most common interventions in medicine.
As seen in Table 2, intravitreal ranibizumab therapy is the intervention of choice for the treatment of subfoveal choroidal neovascularization. Despite the fact that its cost of treatment is the highest among comparators, ranibizumab therapy confers the greatest value of the neovascular macular degeneration therapies. It therefore fulfills Value-Based Medicine Principle 3. 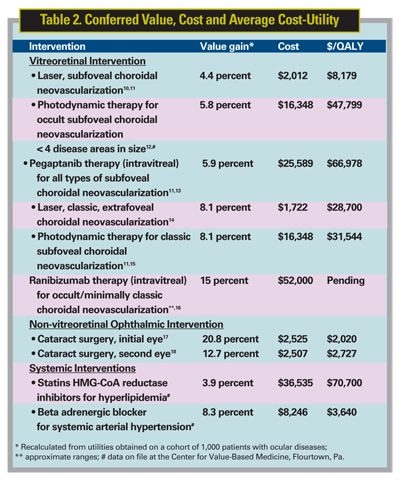
If the treatment of subfoveal occult choroidal neovascular lesions <4 disc areas is under consideration, both intravitreal pegaptanib therapy and PDT statistically confer the same value. This comparison illustrates Value-Based Medicine Principle 4. In this case, PDT is the preferred intervention since it is less costly.
The Future
Standardization of cost-utility analysis is critical for widespread use of the economic instrument. For utility analysis alone, there are more than 800 variants.4 Including other input parameters, there are virtually tens of thousands of different methodologies, thus making most cost-utility analyses incomparable.
Value-based medicine is a powerful tool that will allow the quantification of the exact value of all future interventions for neovascular macular degeneration, whether monotherapy, dual therapy, triple therapy or some other variant. In this article, only interventions well supported by randomized clinical trial data are included. Once available, clinical trial data and other high level, evidence-based data will allow a value-based comparison of the use of bevacizumab (Avastin), the parent drug of ranibizumab, compared to ranibizumab for the treatment of neovascular macular degeneration.
Patients and providers should all welcome value-based medicine. Since it incorporates the best evidence-based data with patient quality-of-life preferences, which are often ignored in primary clinical trial outcomes, value-based medicine allows the clinician to practice higher quality medicine than possible with evidence-based data alone. It can't come soon enough.
The authors manage the Center for Value-Based Medicine and are part of the Leonard Davis Institute for Health Economics,
1. Brown MM, Brown GC, Sharma S, Hollands H, Smith A. Physician manpower and health care expenditures in the
2. Welch WM. Drug prices outstrip inflation.
3. Dorschner J. Drug prices continue to rise. The
4. Brown MM, Brown GC, Sharma S. Evidence-Based to Value-Based Medicine. Chicago, AMA Press, 2005;1-324.
5. Rosenfeld PJ, Brown DM, Heier JS, Boyer DS,
6. Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol 2003;48:204-223.
7. Brown GC, Brown MM, Sharma S. Difference between ophthalmologist and patient perceptions of quality-of-life associated with age-related macular degeneration. Can J Ophthalmol 2000;35:27-32.
8. Brown GC, Brown MM, Brown H, Kindermann S, Sharma S. A value-based medicine comparison of interventions for subfoveal neovascular macular degeneration. Ophthalmology (in press).
9. Gold MR, Patrick DL, Torrance GW, Fryback DG, Hadorn DC, Kamlet MS, Daniels N, Weinstein MC. Identifying and valuing outcomes. In Gold MR, Siegel JE, Russell LB, Weinstein MC (eds). Cost-Effectiveness in Health and Medicine,
10. National Institute for Health and Clinical Excellence (NICE). From the Internet @ www.nice.org.uk, accessed 4-5-07.
11. Brown GC, Brown MM, Sharma S. Incremental cost-effectiveness of laser therapy for subfoveal choroidal neovascularization. Ophthalmology 2000;107: 1374-1380.
12. Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization--verteporfin in photodynamic therapy report 2. Am J Ophthalmol 2001;131:541-60.
13. Gragoudas ES, Adamis AP, Cunningham ET, Feinsod M, Guyer DR for the VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. New Eng J Med 2004;351:2805-2816.
14. Busbee B, Brown MM, Brown GC, Sharma S. A cost-utility analysis of laser photocoagulation for extrafoveal choroidal neovascularization. Retina 2003;23:279-287.
15. Brown GC, Brown MM, Roth Z, Campanella J, Beauchamp GR. The cost-utility of photodynamic therapy in eyes with neovascular age-related macular degeneration. A reappraisal with 5-year data. Am J Ophthalmol 2005;140:679-687.
16. Brown MM. The cost-utility of interventions for neovascular macular degeneration. Present at the
17. Busbee B, Brown MM, Brown GC, Sharma S. Incremental cost-effectiveness of initial cataract surgery. Ophthalmology 2002;109:606-612.
18. Busbee B, Brown MM, Brown GC, Sharma S. A cost-utility analysis of cataract surgery in the second eye. Ophthalmology 2003;110:2310-2317.