Initial presentation and symptomatology to the outside hospital and ophthalmologist prompted a stroke workup. CBC, CMP, ESR,º CRP, Factor V Leiden, Protein C, Protein S, ANA, CCP IGG, anti-dsDNA, Lyme, SS-A antibody, SS-B antibody, echocardiogram, electrocardiogram and MRA were all unremarkable. MRI with tiny foci of T2 hyperintensities scattered in the bilateral cerebral white matter (See Figure 2) elicited a diagnosis of migraine. Given the patient’s history of hypertension, he was started on aspirin 81 mg p.o. daily and cardiovascular risk factors were medically optimized.
On follow-up, the patient’s visual symptoms were stable but headaches were still noted to be prominent, which led to a repeat MRI. This MRI showed bilateral cerebral white matter FLAIR hyperintensities with equivocal interval increase (See Figures 3 & 4). These changes were felt to be consistent with multiple sclerosis. Dilated fundus findings of a retinal hemorrhage, however, led to a fluorescein angiography, which demonstrated a branch retinal artery occlusion in the left eye (See Figure 5) corresponding to that retinal hemorrhage. The patient likely had a subtle branch retinal artery occlusion on initial presentation that was not appreciated on clinical examination. The BRAOs, MRI changes and symptoms of hearing loss with mental status changes led to the diagnosis of Susac’s syndrome.
The patient was started on IV steroids along with an oral steroid taper. He was referred to rheumatology for blood workup and to the Cleveland Clinic’s Susac’s center for appropriate management.
Discussion
Susac’s syndrome is characterized by a clinical triad of encephalopathy, branch retinal artery occlusion and hearing loss.1 It was initially described in 1979, and a recent review article cited that 304 cases have since been reported.2 SS is thought to be an autoimmune disease with unclear pathogenesis in which the small vasculature of the brain, retina and inner ear become occluded. It is three times more common in women compared to men without any racial predilection.3
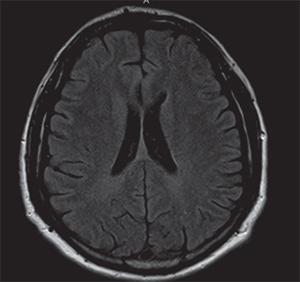 | 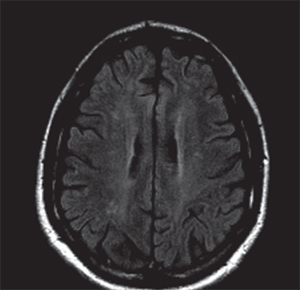 | |
| Figure 2. Axial T2-weighted FLAIR MRI on initial presentation with few areas of hyperintensity scattered in the cerebral white matter thought initially to be due to migraine. | Figure 3. Axial T2-weighted FLAIR MRI from second presentation demonstrating increased white matter hyperintensities compared to the initial MRI (Figure 2). | |
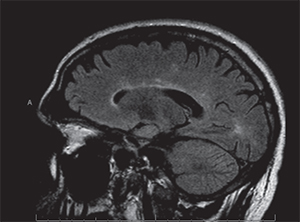 | 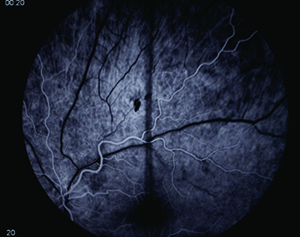 | |
| Figure 4. Sagittal T2-weighted FLAIR MRI on second presentation with areas of hyperintense “spoke” lesions along the roof of the corpus callosum, typical of Susac’s syndrome. | Figure 5. Fluorescein angiography of the left eye demonstrating an arterial filling defect. |
Ophthalmic manifestations of SS arise from the occurence of peripheral branch retinal artery occlusions. These often occur in both eyes, over multiple episodes, and can be either subclinical or result in noticeable decrease in vision. Since the disease often occludes small peripheral arterioles, fundoscopy may be normal with fluorescein angiography being the more sensitive modality for diagnosis.7 Our patient likely had a BRAO on initial presentation causing a visual field defect that was missed by initial dilated eye exam. This suggests that fluorescein angiography may be helpful with routine monitoring of disease status. Spectralis OCT may be used as another diagnostic tool, as those with SS show scattered intraretinal scarring that spares the outer nuclear layer and photoreceptor layers.8
Appropriate treatment of SS is still under debate but many experts agree that acute events in SS may be treated with high-dose IV steroids along with gradual oral taper. Cyclophosphamide and intravenous immunoglobulins are other common choices.9 In general, aspirin is also helpful in these patients as it improves microvascular blood supply.9
In conclusion, Susac’s syndrome is an extremely rare condition but should be considered in patients with branch retinal artery occlusion with auditory and mental status changes. Magnetic resonance imaging, fluorescein angiography and audiology may be helpful in both diagnosis and clinical monitoring. Other ancillary tests including rheumatologic blood testing, visual field testing and OCT may also aid in the diagnosis. Clinical suspicion and appropriate testing are essential as early diagnosis may be important to limiting the sequelae in this clinical entity. REVIEW
The authors acknowledge the assitance of Adam Flanders, MD.
1. Susac J. Susac’s syndrome: The triad of microangiopathy of the brain and retina with hearing loss in young women. Neurology 1994;44:591-593.
2. Greco A, De Virgilio A, Gallo A, Fusconi M, et al. Susac’s Syndrome—pathogenesis, clinical variants and treatment approaches. Autoimmunity Reviews 2014;13(8):814-821.
3. Susac J. Susac’s syndrome. Am J Neuroradiol 2004;25:351-352.
4. Minor L, Schessel D, Carey J. Meniere’s disease. Curr Opin Neurol 2004;17:9-16.
5. Buzzard K, Reddel S, Yiannikas C, Sean Riminton D, et al. Distinguishing Susac’s syndrome from multiple sclerosis. J. Neurol 2015;262(7):1613-21.
6. Susac J, Murtagh F, Egan R, Berger J, et al. MRI findings in Susac’s syndrome. Neurology 2003;61:1783-1787
7. Papo T, Biousse V, Lehoang P, et al. Susac syndrome. Medicine 1998;77:3-11.
8. Ringelstein M, Albrecht P, Kleffner I, Bühn B, et al. Retinal pathology in Susac syndrome detected by spectral-domain optical coherence tomography. Neurology 2015;85(7):610-8.
9. Gross M, Eliashar R. Update on Susac’s Syndrome. Curr Opin Neurol 2005;18(3):311-314.



