Radouil T. Tzekov, MD, PhD, and Amanda Howe,
Instead of waiting for a disorder to occur and then treating it with a drug, imagine if we could prevent it from ever happening in the first place. This is the promise of gene therapy, and it's a new frontier for ophthalmic therapies that will be increasingly relevant in the decades to come. As appealing as prevention is, however, gene therapy's efficacy can be very difficult and costly to demonstrate, as it requires large numbers of patients enrolled in long-term studies. Nevertheless, great strides are being made in the development of gene therapy treatment methods, including vector choice, targeting, selectivity and stability.
The gene therapy methods known as gene delivery and RNA interference (RNAi) are both being investigated in humans and animals. Given the relative immune privilege afforded by the blood-retina barrier, the eye is a desirable organ for this revolutionary research, although it's not free of complications. Even though this therapy is still far from the mainstream, here's a look at its exciting implications.
Gene Therapy and the Eye
Many fears surround gene therapy, stemming from concerns about inadvertently activating an autoimmune response, transfecting the wrong cells, activating oncogenes, or stimulating viral disease or tumor growth. While the blood-retina barrier provides some protection against such mishaps and decreases the likelihood of transfected cells spreading systemically, there's no guarantee.
When other areas of the body receive gene therapy, rejection of viral capsids carrying the payload is a concern. The eye however, experiences reduced chances of vector rejection,1 just as foreign tissue in the eye experiences extended, if not indefinite, survival where it would be rapidly rejected in other parts of the body.2
The eye is also relatively easy to monitor, both for potential side effects and for treatment benefit, with methods such as electroretinography, visual acuity, optical coherence tomography and perimetry. Ophthalmoscopy and fundus photography also provide an easy way to directly visualize and document therapeutic effects.
Many gene therapy techniques have been investigated, but virus-based approaches are moving quickly through the pipeline.3
Viral Carrier Pigeons
The metabolic properties of a virus are inert outside a cell but, upon encountering host cells, the virus is able to co-opt some functions from the host and exploit them in place of the reproductive machinery it lacks. These actions enable viral replication and perpetuate the infection.4 Viruses generate messenger RNA (mRNA) to transport the genetic information of the intended processes from their nucleic acid, encapsulated in capsids, to the host's ribosomes.4 Therefore, human alteration of virus nucleic acid can modify the end product of this process, and transform viruses from malevolent microorganisms to compliant partners, if all goes according to plan.4 For the purposes of gene therapy, the virus is stripped of its own destructive DNA and genes, and the desired genes are inserted instead. Vectors don't carry the proteins themselves; rather, the payload is the genetic material necessary to take over the cell's protein manufacturing processes and coerce it to produce the desired proteins.
Certain diseases result from miscommunication in which the DNA transcription provides faulty production information to the cell; in other instances, the information is missing.
Delivering a backup plan to the nucleus of a cell, however, is an insurmountable task for all but one organism: the virus. Gene therapy first began by using plasmid-based systems, but these were eventually replaced with retrovirus vectors.5 Retroviruses, however, soon displayed limitations: they require active cell division for gene transduction; possess significant oncogenic potential and have been implicated in unwanted gene silencing side effects.5 Some, such as lentiviruses, carry a greater risk of mutagenesis.6 Baculovirus vectors are known for their large payload capabilities and low cytotoxicity, but are not the most popular for use in the eye,7 though they're used in stem cell engineering.
Adeno-associated viruses have become the preferred vector. Wild-type AAVs are not implicated in disease— only mild immune responses—when unaccompanied by a "helper" adenovirus to co-infect cells. AAVs also enjoy a broad host range as they can infect both dividing and non-dividing cells, and can be pseudotyped to create more than 100 chimeras, or facades, that can deceive host cells that would otherwise resist transfection.2 By propagating viruses that lack a specific complement or other factor that would be cause for alarm to the target cell, the virus can become a better infiltrator.3
Furthermore, different AAV serotypes can package different numbers of cassettes, the manipulated fragments of DNA. Serotype AAV5 capsids have the largest capacity.8
Finally, AAVs can also integrate into host chromosomes or exist autonomously in the cytoplasm, so that they have the potential for long-term expression.5
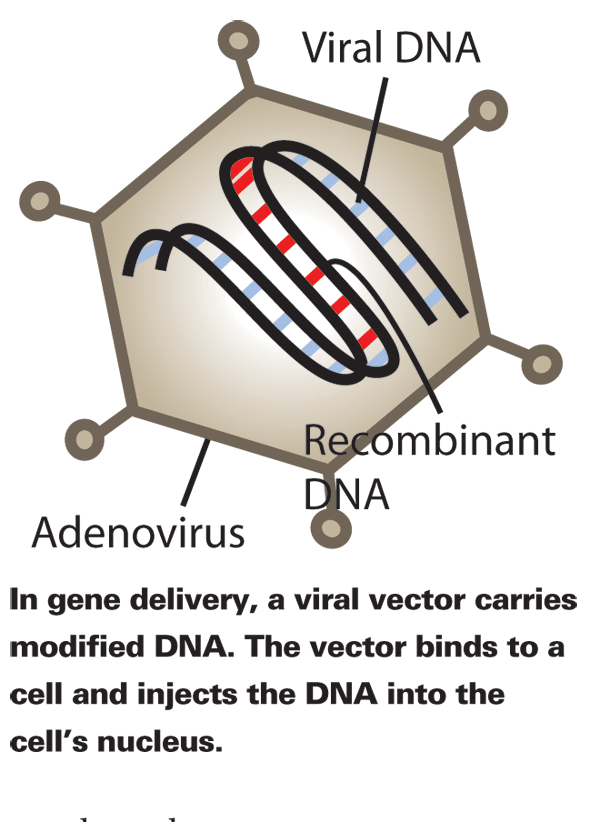
Targets and Methods
Today, there are two major modes of gene therapy undergoing research. First, there's gene delivery, which is in the early stages of clinical development.9-11 In gene delivery, vectors carrying genes transfect cells, augmenting underexpressed genes or replacing mutated ones. Having completed phase II and entered phase III studies, RNA interference (RNAi) technology is further along in development.11 This method silences genes by inhibiting the accessibility of DNA to transcriptional machinery.
• Single-gene transfection (gene delivery). The blood-retina barrier partition and pharmacokinetic properties of the retina make it attractive for gene therapy. Also appealing is the number of genetic conditions of the retina, which number more than 25.12
One particular condition for which gene delivery is being investigated is Leber's congenital amaurosis. A group of inherited, blinding conditions that present during childhood and progress over time, LCA often results in blindness. One form of the disease, known as LCA2, caused by mutation of the retinal pigment epithelium gene RPE65, is a major target for gene therapy.13
By stimulating production of the RPE65 protein through gene delivery, it's postulated that LCA2 can be reversed. Beginning in 2007 at
Most patients self-reported increased visual sensitivity in the study eye compared with their control eye, especially under reduced ambient light conditions. Visual acuity was also generally stable and even improved in five patients.13-15
Another target for gene delivery is age-related macular degeneration. The number of Americans with early-stage (dry) AMD is estimated to be about 7.3 million, with around 1.75 million having advanced (wet) AMD.16 Some patients with wet AMD respond to anti-angiogenic compounds, but gene therapy represents a more inclusive treatment option, since it may be more effective to prevent the expression of angiogenic factors at the transcriptional level than to inhibit the vascular endothelial growth factor signaling cascade with anti-VEGF treatments.
Therapeutic inhibition of angiogenesis could inhibit ocular neovascularization associated with excessive levels of VEGF, the characteristic feature of both wet AMD and diabetic retinopathy. VEGF must bind to receptors to stimulate the proliferation of new blood vessels. In 2002, a mouse model of ischemia-induced retinal neovascularization was used to research the method of AAV delivery to VEGF receptor flt-1, and ways to block it. Doing so has been shown to reduce the number of endothelial cell nuclei in the retina by about half.17 Similar results were obtained later in a rat model that closely mimicked human retinopathy of prematurity.18
• RNA interference (gene silencing). Conditions that stubbornly refuse to be affected by available medications can be thwarted by "switching off" the genes that encode defective proteins. Partitioned organs such as the eye, lungs and central nervous system are being closely investigated for the applicability of RNAi.
RNAi is used as a method of inhibiting genes which encode vascular endothelial growth factor and other similarly acting molecules. The process of RNAi destroys the genes using the double stranded RNA (dsRNA) of carrier viruses. The dsRNAs are cut into small, 21- to 23-piece nucleotides called short interfering RNA (siRNA) by a Dicer enzyme. Then, a protein called RNA-induced silencing complex (RISC) "unzips" the siRNA, removes and discards the targeted strand, and degrades the mRNA indicated on the siRNA so that it's no longer replicated.19 RNAi can suppress virtually any gene, and is being studied in many contexts.2,20,21
Hurdles to Overcome
As promising as the various modes of gene therapy are, they are subject to multiple obstacles. Economic barriers of all sorts hinder the progress of research. In particular, orphan diseases (where genetic causes are better identified) frequently fall victim to high development costs compared to the treatment's potential profitability. The resulting high treatment costs discourage insurance coverage. Public funding through the National Institutes of Health, the National Eye Institute and others has played a crucial role in the development of treatments like the AAV2 RPE65 treatment,22 and will continue to do so, but private philanthropy also plays a key role, as well. Organizations such as the Foundation Fighting Blindness, created in large part by Gordon Gund, have been great boons to research.23
In order for people to be enrolled in clinical trials in retinal gene therapy, they must need treatment for a sight-threatening disease and have an acceptable risk-to-benefit ratio.
Although in vitro experiments and animal models can provide substantial preliminary information, human trials are necessary to fully understand and validate the therapy. For example, once clinical trials are in progress, biological obstacles could begin to manifest.
Most diseases result from multiple gene mutations; currently it's impossible to target all of them at once. In patients with retinitis pigmentosa, for example, to treat the entire afflicted population more than 30 genes must be targeted.12
Due to difficulties in conducting human studies, many studies published in 2009 have focused on animal models. However, most pre-clinical results are promising and provide hope for clinical application in the near future.
Although many hurdles remain, gene therapy for retinal diseases is one of the more exciting experimental therapeutic approaches in development. We expect the progress in this area to continue and clinical trials to begin in diseases more prevalent than LCA.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Streilein JW. Ocular immune privilege: The eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol 2003;74:2:179-85.
2. Grimm D, Kay MA. RNAi and Gene Therapy: A Mutual Attraction. Hematology Am Soc Hematol Educ Program 2007;2007:473-81.
3. Kost TA, Condreay JP. Recombinant baculoviruses as mammalian cell gene-delivery vectors. Trends Biotechnol 2002;20:4:173-80.
4. Madigan MT, Martinko JM. Biology of Microorganisms. 11 ed.
5. Tomar RS, Matta H, Chaudhary PM. Use of adeno-associated viral vector for delivery of small interfering RNA. Oncogene 2003;22:36:5712-5.
6. Yanez-Munoz RJ,
7. Kinnunen K. Baculovirus is an efficient vector for the transduction of the eye: Comparison of baculovirus- and adenovirus-mediated intravitreal vascular endothelial growth factor D gene transfer in the rabbit eye. J Gene Med 2009;11:5:382-9.
8. Allocca M, Doria M, Petrillo M, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest 2008;118:5:1955-64.
9. Clinicaltrials.gov. Phase 1/2 safety and efficacy study of AAV-RPE65 vector to treat LCA. http://www.clinicaltrials.gov/ct2/show/NCT00749957?term=NCT00749957&rank=1. (June 11, 2009).
10. Clinicaltrials.gov. Phase I trial of gene vector to patients with retinal disease due to RPE65 mutations (LCA). http://clinicaltrials.gov/ct2/show/ NCT00481546?term=NCT00481546&rank=1.
11. Clinicaltrials.gov. Safety and efficacy study evaluating the combination of bevasiranib and lucentis therapy in wet AMD (COBALT). http://www.clinicaltrials.gov/ct/show/NCT00499590?order=1.
12. RetNet. Summaries of genes and loci causing retinal diseases. http://www.sph.uth.tmc.edu/retnet/sum-dis.htm#A-genes. June 18, 2009.
13. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber"s congenital amaurosis. N Engl J Med 2008;358:21:2240.
14. Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of LCA due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum Gene Ther 2008;19:10:979.
15. Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber"s congenital amaurosis. N Engl J Med 2008;358:21:2231-9.
16. Friedman DS, O"Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the
17. Bainbridge JW, Mistry A, De Alwis M, et al. Inhibition of retinal neovascularisation by gene transfer of soluble VEGF receptor sFlt-1. Gene Ther 2002;9:5:320-6.
18. Rota R. Marked inhibition of retinal neovascularization in rats following soluble-flt-1 gene transfer. J Gene Med 2004;6:9:992-1002.
19. Novina CD, Sharp PA. The RNAi revolution. Nature 2004;430:6996:161-4.
20. Clinicaltrials.gov. Safety study of CALAA-01 to treat solid tumor cancers. http://clinicaltrials.gov/ ct2/show/NCT00689065?term=RNAi&rank=1. (June 11, 2009)
21. Clinicaltrials.gov. Dose escalation trial to evaluate intravenous ALN-VSP02 in patients with advanced solid tumors with liver involvement. http://clinicaltrials.gov/ct2/show/NCT00882180?term=RNAi&rank=2.
22. Jacobson SG, Boye SL, Aleman TS, et al. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther 2006;17:8:845-58.
23. Avril T. Venture capitalist Gordon Gund funds research.



