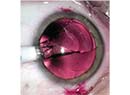
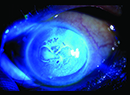
As diagnostic and surgical technologies have evolved and IOL power-calculation formulas have become ever more sophisticated, cataract surgery has become a form of refractive surgery. Today’s patients expect excellent vision postop, and for the most part that’s what they get. However, the reality is that some practices routinely produce better outcomes than others, which indicates that there’s still room for improvement in the way surgeons and practices manage these patients.
In the past, surgeons were happy to get patients within 1 D of the refractive target; today, the goal has largely shifted to getting patients within 0.5 D—especially when the patient is paying out-of-pocket for an advanced-technology lens. Terrence P. O’Brien, MD, a distinguished professor of ophthalmology and the director of the Refractive Surgery Service at Bascom Palmer Eye Institute of the Palm Beaches, notes that even with all of today’s technology, outcomes only fall within that tolerance about half of the time. “That indicates that perhaps we’re not performing as well as we think we are with clinical outcomes,” he says.
Here, Dr. O’Brien and other surgeons offer strategies you can use to upgrade your outcomes by improving your practice philosophy, patient exams, preoperative patient management, power-calculation formula usage, surgical technique and postoperative patient management.
Setting the Process in Motion
If you’re serious about getting more of your patients within 0.5 D of your target refraction, the first thing to do is look at the big picture:
• Make improving your outcomes a practice priority. Steven C. Schallhorn, MD, a professor of ophthalmology at the University of California San Francisco, chief medical director for Zeiss Meditec, and in clinical practice in San Diego, notes that simply saying you’d like your outcomes to improve is a far cry from actually putting in the time and effort to make it happen. “We all want better outcomes, but actually improving outcomes has to be a practice priority,” he emphasizes. “It takes commitment, resources and enlisting the assistance of your staff. This is about putting your money where your mouth is.”
• Make teamwork central to your process. R. Bruce Wallace III, MD, FACS, founder and medical director of Wallace Eye Associates in Alexandria, Louisiana, and clinical professor of ophthalmology at Louisiana State University and Tulane Schools of Medicine in New Orleans, notes that his practice has been highly focused on getting patients within 0.5 D of target for many years. “One of the biggest lessons we’ve learned is that accuracy in getting your outcome close to your target depends on teamwork,” he says. “Our biometrist, Marsha Hesni, is a fully certified ophthalmic technician. She and Robert Crotty, OD, our clinical director, work together with me to make sure our measurements are accurate and spot any anomalies. Dr. Crotty sees our cataract patients postoperatively, so he’s well aware of how each patient does and what issues may be involved if we miss the target. Ms. Hesni and Dr. Crotty have significant experience with these processes. It wouldn’t make sense for me to not take advantage of that.”
“We’re all human,” adds Dr. Crotty. “We can all make mistakes and fail to see something. This gives us three opportunities to look over the measurements. If something looks different or strange, hopefully one of us will pick up on that and we’ll repeat the measurements, if necessary, so we get the best possible outcome.”
• Simplify your processes to reduce the likelihood of human error. “As much as we don’t like to admit it, there are many possible sources of human error,” notes Dr. Schallhorn. “We want to minimize any possibility of having these type of errors, because any error in translation, transcription or capture can result in a dramatically deviant outcome.
“In general, more straightforward and simplified processes result in less chance of human error,” he continues. “Make your clinical and surgical procedures standardized and systematic, and eliminate unnecessary steps. For example, to avoid transcription errors, don’t require that the biometry be handwritten onto a form, only to then be manually entered into a power calculation formula. That represents two opportunities for an entry error.”
Getting Accurate Measurements
These strategies can help you get more accurate preop measurements:
• Have your best, most trusted technician perform the biometry. “Everyone appreciates the value of good biometry—in theory,” says Dr. Schallhorn. “But in practice, the way to obtain high-quality, repeatable measurements is to have your most trusted technicians doing the biometry. Furthermore, you want to be sure that person is well-trained, especially in terms of knowing the difference between a good capture and a not-so-good capture. This involves analyzing multiple captures, ensuring proper alignment and fixation, comparing left and right eye measurements, and checking keratometry values from other devices.”
• Confirm the quality of your biometry. Daniel H. Chang, MD, a partner at Empire Eye and Laser Center in Bakersfield, California, points out that when it comes to preoperative biometry, there are two crucial aspects to consider. “First, you need good-quality measurements,” he says. “Second, you need a way to assess their quality. Topography and OCT can help you do that.
“This additional testing is important,” he notes. “For example, to correct astigmatism, you need good keratometry—but you also need to know if the astigmatism is regular or irregular, so you should perform topography. Technically, you only need the keratometry values for your formula, but there’s no way you can know the relevance or quality of your keratometry without a topographic map.”
• Take your biometry measurements before drops are instilled. “Drops can alter the accuracy of the measurement,” Dr. Chang points out.
• Don’t worry about measuring with multiple biometers. “Multiple devices mean more data,” says Dr. Chang. “But if two different machines give you different results, you have to repeat all measurements and look for intra- and inter-measurement variability, as well as intra- and inter-device variability. I prefer to assess repeatability by taking multiple measurements with the same device, usually on separate days. A modern device like the IOLMaster or Lenstar can give great outcomes, especially if you take the time to work at improving your processes.”
Dr. Schallhorn agrees that surgeons probably don’t need measurements from multiple biometry devices—at least for the average, uncomplicated cataract patient. “There are patients for whom having multiple biometry measurements could be of value,” he says. “However, for the majority of straightforward cataract procedures, I don’t believe it’s necessary. Frankly, using multiple devices is inefficient and may create more problems in a busy practice with high staff turnover. I don’t think that’s going to help drive better outcomes in the vast majority of uncomplicated cataract procedures.”
| Should You Customize Your A-constant? | ||
|
• Remember that lane length can affect your refractive measurements. Dr. Chang points out that the refractions may need to be adjusted to the length of the exam lane. “We’re used to adding a ‘reading add’ to our distance refraction for near correction, but in our limited-length exam rooms we may also need to do this for distance,” he explains. “At 13 feet, we’re 0.25 D from optical infinity, and even at a full 20 feet—either direct or mirrored—we’re off by 0.16 D. In fact, variabilities in A-constant normalization may be related in part to lane length. The difference between a 20-foot lane and a 10-foot lane can be a third of a diopter—the same variability that we see between A-constants from different surgeons.”
Dr. Chang says that despite the fact that the absolute difference the compensation for optical infinity makes is small, it has affected some of his patients. “I have patients who are 20/15 in the room after cataract surgery,” he says. “They say, ‘I can see the eye chart, but I can’t see the street sign a few hundred yards down the road.’ Compensating for my 7.5-foot lane with a -0.5 D lens sharpens up the far distance. Many patients wouldn’t notice this, but if you have a demanding patient who is 20/20 in the room, the extra 0.25 or 0.5 D can make a difference.”
• Know when the outcome is less certain, and alert the patient. “We do our best to hit the target, but it’s very valuable to know whether hitting the target is likely or unlikely,” Dr. Chang explains. “Then we can prep the patient, so an imperfect result won’t be a surprise.
“There have been times when I’ve done two or three measurements on different days, and I don’t get a consistent reading,” he continues. “When that happens I tell the patient that the ocular surface isn’t stable, so we’ll need to treat before proceeding with surgery. Furthermore, if the patient is paying extra for a premium lens, I may change the plan. I may opt for a basic monofocal lens and prepare the patient for glasses postoperatively.
“In some cases, I may still opt for an extended-depth-of-focus lens,” he adds. “However, I tell the patient that he’ll have some fluctuation in vision. Patients understand that and expect to have some issues postoperatively.”
Managing the Ocular Surface
“Ocular surface disease is probably the greatest single factor that potentially interferes with the accuracy of our biometry,” notes Dr. O’Brien. “This is one of the most common reasons for patient dissatisfaction and the IOL power being off target. Sometimes if you’re implanting a toric, the unstable ocular surface will also lead to incorrect axis determination and alignment.
“To maximize your outcomes, you need to be addressing the ocular surface before proceeding with surgery,” he says. “That’s often a problem, because patients want to get in and have surgery performed promptly, so offices tend to streamline everything to get the patient to the OR quickly. But when a patient has a problem with the ocular surface, we need to step back and delay surgery for four to six weeks until treatment measures can lead to an improved, more stable ocular surface and tear film.”
Dr. O’Brien observes that when a cataract patient has an ocular surface problem, it’s usually for one of two reasons. “Sometimes the problem is caused by prescription eye drops, such as preserved glaucoma medications,” he says. “Those can be very rough on the ocular surface either because of some intrinsic cytotoxicity or the effect of preservatives, or both. This is especially likely to happen when the patient is using multiple glaucoma agents up to three or four times a day.
“There are several ways to address this,” he continues. “One approach is to talk to the glaucoma subspecialist who’s managing the patient and ask if the medications can be adjusted. Perhaps the patient can switch to preservative-free medications. Three are currently available: timolol; timolol-dorzolamide; and tafluprost. Another possibility would be to perform selective laser trabeculoplasty, which could reduce the patient’s need for medications. A third option would be to replace some or all of the drops with a short-term course of an oral carbonic anhydrase inhibitor such as acetazolamide or methazolamide. Of course, this has to be done with care, because systemic medications can have significant systemic side effects. I would do at least one of these things preoperatively and then give the ocular surface four to six weeks to improve.
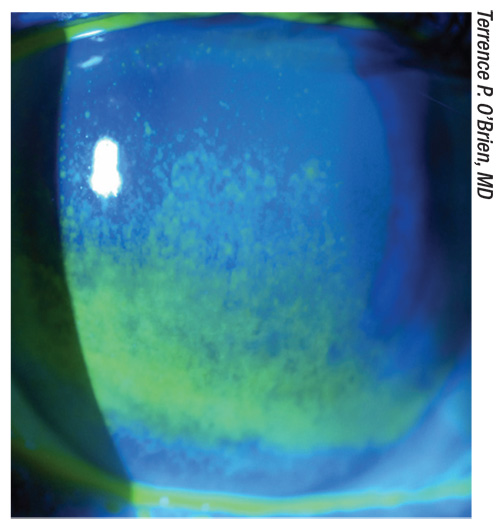 |
| A preoperative cataract patient with glaucoma and an unstable tear film that need to be addressed prior to surgery. |
“The other type of patient who commonly presents with cataract and a less-than-ideal ocular surface is a senior with dry-eye disease,” Dr. O’Brien notes. “A study by William Trattler and colleagues found that 87 percent of patients presenting for cataract surgery had significant dry-eye disease.1 Often this is underdiagnosed or not properly addressed. For these patients we initiate an aggressive treatment plan to rapidly stabilize the ocular surface. Usually that involves a course of a topical corticosteroid, sometimes also starting an immunomodulatory agent such as cyclosporine-A or lifitegrast. In this situation we have a preference for starting lifitegrast because it has demonstrated a more rapid onset of action than cyclosporine-A, which may take several weeks or even months to achieve optimal effect. Lifitegrast has been shown to take effect within two to three weeks. That’s more likely to help in this preoperative situation with acute therapy.”
Of course, there are many different causes for dry-eye disease. “If the patient has a meibomian gland dysfunction, we have a low threshold for performing thermal pulsatile therapy with LipiFlow,” he says. “The other modality that’s been helpful for post-LASIK cataract patients and select others is neurostimulation using the TrueTear device, which is put up the nose to electrically stimulate the trigeminal nerve. It’s been shown to increase all three layers of the tear film—aqueous, lipid and mucin.
“The main thing is to make sure you notice an ocular-surface problem and take the time to address it in order to let the surface heal before proceeding with surgery,” he concludes. “That will go a long way toward improving your IOL power calculations and refractive outcomes.”
Calculating the Parameters
Once you have accurate measurements, these strategies will help ensure good outcomes:
• Use a current formula. “This is important because older formulas are not as accurate for long or short eyes,” says Dr. Chang. “Ten years ago when we looked at the best formulas of the time, we found that the SRK/T, Holladay I and II and Hoffer Q all work pretty well for average-length eyes—but for really long or short eyes, none of them did that well.2 Basically, the farther an eye deviates from average values, the greater the variability when using the older formulas. In contrast, the newer formulas, such as Barrett II and Hill-RBF, can give excellent results over a range of eye measurements.”
• Once you choose a formula, stick with it. “Don’t needlessly and repeatedly switch power-calculation formulas, because that will make it more difficult to extract useful data from your postop outcomes,” Dr. Schallhorn points out. “If you do change your formula, make sure you stick with it long enough to be able to derive meaningful outcome data.”
• If an eye isn’t average, try running the numbers through multiple formulas. “Sometimes when you have a more challenging eye, such as a very short eye or an eye that’s had previous refractive surgery, it’s helpful to try different formulas,” says Dr. Crotty. “Certain formulas are known to work well with certain eyes, so those formulas may swing your decision one way or the other. Furthermore, circumstances may make one formula less likely to produce a great outcome. For instance, the Holladay II formula uses some measurements taken from the refraction. Sometimes the cataract can alter the refraction, throwing off your calculation. So in that situation you might decide to look at the Barrett Universal formula as well, just to give you another perspective.”
Dr. Crotty adds that some formulas work better in non-average eyes when used with specific mathematical adjustments. “To make the most of these formulas you need to know when the eye isn’t standard, and what adjustments may be needed to ensure a good outcome,” he says.
• Remember to account for the refractive impact of the posterior cornea. “Thanks to seminal studies by Doug Koch, MD, and others, we have a renewed appreciation of the influence of posterior corneal optical power,” notes Dr. Schallhorn. “We used to make very simple assumptions about the posterior corneal power. Today, we know that those assumptions can lead to unsatisfactory outcomes, particularly in patients with previous laser vision correction, or when implanting a toric IOL. Incorporating a more accurate assessment of the overall corneal power can help us further improve the predictability of refractive outcomes.”
• When adding your surgically induced astigmatism to online toric calculators, use postoperative keratometry data—not refraction data. “Surgically induced astigmatism accounts for the aggregate effect of the surgical procedure on the patient’s corneal astigmatism,” Dr. Chang notes. “This number becomes important when trying to fine-tune toric IOL calculations. Traditionally, SIA is calculated from the postoperative refraction, but these measurements include the effect of posterior corneal astigmatism, which can vary from eye to eye. Since toric calculators (and some biometers) separately account for posterior corneal astigmatism, you should calculate your SIA using postoperative keratometry. A simple keratometric measurement with the IOLMaster or Lenstar postoperatively will give you that information.”
Maximizing Surgical Results
These strategies can help minimize negative impacts from your surgery:
• Don’t ignore astigmatism. “You have to get astigmatism below 0.25 D in order for patients to be very happy with their outcomes,” says Dr. Crotty. “In the past surgeons primarily used manual limbal relaxing incisions, but today surgeons have a number of tools, including toric IOLs and femtosecond lasers, that allow them to hit the target more precisely.”
Dr. O’Brien points out that having a femtosecond laser to make arcuate incisions can improve the accuracy of their effect. “The problem with manual arcuate keratotomy incisions is their inconsistency in terms of length, shape and depth,” he says. “A femtosecond laser can ensure that the incisions are placed exactly where you want them, and with some devices now offering real-time OCT, we can check the precise depth of the incision as it’s created. And of course, a laser can create a more accurate and reliable capsulotomy, in terms of shape, size and position, which—in theory at least—may help to ensure that the lens ends up exactly where you expect it to be. I think this is especially valuable when you’re implanting an advanced-technology IOL.”
| Should You Repeat Biometry on Different Days? | ||
|
Dr. Wallace adds that he’s implanting more toric IOLs than ever. “Even lower powers like 0.75 D are helping us to get better visual results,” he says. “Interestingly, depth of field seems to be better with these lenses. Patients seem to have better intermediate vision, as well as better distance vision.”
• Whenever possible, make your incision temporal, at the limbus. “There are good surgeons who make superior or superior-nasal/temporal incisions,” notes Dr. Chang, “but the cornea is longer temporally, so I like to make my incisions there. If you’re careful, a well-constructed tri-planar, sub-3-mm, temporal incision at the limbus should induce less than 0.1 D of astigmatism.”
• If you don’t have zonular integrity, consider implanting a three-piece lens. Dr. O’Brien points out that weak zonules can allow the lens position to shift, altering the refractive outcome. “If an eye has stable zonules and an intact capsulorhexis, a single-piece lens might provide superior centration and optical properties,” he says. “However, if there’s weakness in the zonules, or the capsulorhexis is incomplete or irregular, sometimes a three-piece lens can give you added support that will allow it to remain in a stable position and not become decentered, which a single-piece lens might do under those conditions. For example, a patient with pseudoexfoliation syndrome might be predisposed to weak zonules. If you were planning to implant a single-piece lens, you might want to alter your plan to err more on the side of support and stability.”
• Make sure the optic is surrounded 360 degrees by an overlapping rim of anterior capsule tissue. “Most surgeons understand that this helps to stabilize the optic,” says Dr. Wallace. “One way to accomplish this is to mark the cornea with a 6-mm-diameter circle prior to making the capsulotomy; then you use that as a visual template, staying inside that circle while making the capsulotomy. Factoring in corneal magnification, that will help you create a 5-mm capsulotomy. When you finish the case, you’ll have a 6-mm lens optic with a half-millimeter rim of anterior capsule coverage all the way around.”
• Consider using intraoperative aberrometry. Some surgeons use this technology for assistance in selecting the right lens, and feel that it can be particularly helpful in challenging eyes (such as those with prior refractive surgery) and eyes receiving a toric lens, where precise positioning is crucial. One study using the Optiwave Refractive Analysis System (ORA) found that outcomes were significantly more accurate with the system (p<0.0001), with a mean absolute error of 0.42 D. Sixty-seven percent of eyes were within ±0.5 D of target; 94 percent were within ±1.0 D.3
• Make sure the lens is centered properly before finishing the surgery. “Sometime near the end of a difficult case, the pupil starts coming down,” says Dr. Wallace. “It’s tempting to assume all is well and just finish the case, but it’s not that unusual for the lens to be a little decentered; maybe one of the loops is not in the bag. In that situation you need to investigate before finishing. If I’m not certain where the optic and loops are, I’ll stop and refill the eye with viscoelastic and push the iris around a little bit to make sure the lens is in the bag.”
In fact, centering the lens may be especially important when the lens is aspheric. “The greater the asphericity of the lens, the more important centration is,” Dr. Crotty points out.
• Remove the viscoelastic from behind the optic, not just in front. “In the long term, it’s possible that this won’t make too much difference in the patient’s vision, but in the short term it might,” says Dr. Wallace. “I also find this helpful for accurately assessing the patient before I finish the case.”
Gathering Postop Data
To improve your outcomes, you have to track them.
• Be diligent about collecting data postoperatively. “The critical elements are accurately measuring and electronically recording postoperative information,” says Dr. Schallhorn. “This means performing a refraction on all patients at a consistent postoperative time interval. Find out what percent of your patients are within a half-diopter of target at one month, and check their level of unaided vision, satisfaction with the outcome, and so on. Don’t fall into the trap of not refracting patients because they’re happy.”
• Take your outcome measurements at four to six weeks postop. Dr. Schallhorn notes that the timing for the follow-up measurements involves a bit of compromise. “You’re more likely to get patients to return for follow-up sooner after surgery,” he says. “On the other hand, the longer you wait, the more likely the eye will be stable. For A-constant adjustment, refracting patients at one day or even one week is too early. I’ve found that almost all patients will return for an examination at one month postop, but the return rate drops off after this. Fortunately, by four weeks postop, almost all patients are refractively stable, so this represents a reasonable compromise for data analysis.”
The Journey Continues
Dr. Schallhorn notes that cataract surgery refractive outcomes are considerably better than they were in years past. “Nevertheless, there’s plenty of room for improvement,” he says. “As Graham Barrett stated, we should be getting 90 percent of our uncomplicated cataract procedure patients within a half-diopter of our intended target. We’re not there yet, so we need to keep striving to do better.” REVIEW
Dr. Schallhorn is chief medical officer for Carl Zeiss Meditec. He has no other financial ties to any product mentioned. Dr. Chang is a consultant for Carl Zeiss Meditec and Johnson & Johnson Vision. Drs. Wallace, Crotty and O’Brien have no financial ties to any product discussed.
1. Trattler WB, Majmudar PA, Donnenfeld ED, et al. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: The effect of dry eye. Clin Ophthalmol 2017;11:1423-1430.
2. Narvaez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg 2006;32:12:2050-3.
3. Ianchulev T, Hoffer K, Yoo S, et al. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery. Ophthalmology 2014;121:1:57-60.