|
"We're definitely reaching a point of critical mass in [genomic] research ... now our perseverance is ready to pay off."
–Janey Wiggs, MD, PhD |
Imaging at the Cellular Level
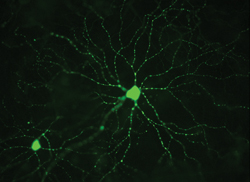
One of the most interesting and potentially significant areas of research in glaucoma today is the use of increasingly sophisticated imaging technology to reveal more about the structural changes that accompany glaucoma at the cellular level.
“The technology available to image retinal ganglion cells has advanced enormously over the past few years,” says Keith Martin, MA, DM, MRCP, FRCOphth, professor of ophthalmology at the University of Cambridge and Clinical Director for Ophthalmology at Cambridge University Hospitals NHS Foundation Trust. “Some of the work that’s going on in the research lab has allowed us to see structural details we’ve never been able to see before. In fact, there’s now some evidence that using advanced imaging techniques to evaluate the retinal ganglion cell complex could be predictive of future functional loss.
“Our lab is using high-resolution imaging techniques to see what happens to retinal ganglion cells, in particular their dendritic trees, when they’re injured in glaucoma,” he continues. “In animal models we can now fluorescently label ganglion cells using various techniques, and we can observe, in vivo, changes that occur longitudinally over time. For example, we’ve learned from experimental models that one of the things that goes wrong in early glaucoma is axonal transport—the movement of stuff back and forth from the retina to the brain along ganglion cell axons. If we can find a way to image axonal transport in real time in humans—something we can already do in animal models—that might provide a readout of the health of the ganglion cells across the retina.
“Basically, we’re interested in identifying markers for cell sickness rather than cell death,” he says. “We can identify a cell that is undergoing apoptosis, but that cell is already effectively dead and will disappear shortly. On the other hand, some of the early changes that occur when a cell is put under stress are reversible, and what we’re looking for is rescue, not just counting dead bodies.”
Dr. Martin notes that this technology could make a big difference in clinical trials of potential neuroprotective drugs. “The neuroprotection trials in glaucoma have been very long and expensive because visual fields are a highly variable outcome measure,” he says. “It takes a long time to see a protective effect. What we’d like is something more sensitive, something that will tell us whether our treatments are having a beneficial effect on cells in a more timely and cost-effective manner.
“Unfortunately, right now we don’t have any good way to image retinal ganglion cells or their dendrites in humans,” he notes. “We don’t want to be doing fluorescein injections in human eyes, and some of the best animal models are transgenic animals that express fluorescent proteins, which is not a viable model for humans. However, many researchers are working on ways to use these imaging technologies in human eyes.
“It’s an exciting time to be in this field,” he concludes. “There’s a lot going on and we’ve got fantastic tools. I can’t think of any other area of neuroscience or medicine where we’ve got the ability to directly observe relevant processes at cellular and subcellular levels in detail, in the living organism.”
Uncovering the Genetics of Glaucoma
Another area that holds great potential for revolutionizing glaucoma diagnosis and treatment is the study of genetics. Janey Wiggs, MD, PhD, Paul Austin Chandler Associate Professor of Ophthalmology at Harvard Medical School and associate director of the Ocular Genomics Institute in Boston, explains why research in this area started slowly but is now reaching critical mass.
“About 20 years ago people started working with the genetics resources that were available, which meant using genetic linkage analysis with families who had early-onset glaucoma,” says Dr. Wiggs. “That was pretty slow going, but this work did identify a handful of genes that cause early-onset types of glaucoma. However, to identify genes influencing adult-onset disease we needed new approaches and larger collections of cases and controls.”
Dr. Wiggs lists a number of factors that are now producing much more information at a much faster rate:
• Completion of the human genome sequence and map. “In 2003 the human genome sequence was completed,” she says. “That was very important because it created a map of many markers throughout the human genome. Those markers—specifically, the single nucleotide polymorphisms, or SNPS—made it possible to evaluate regions of the human genome for genetic associations. This was followed by the completion of the HapMap in 2005, which provided a roadmap for the thousands of SNPs that had already been discovered. With the map in hand, we were able to start doing things like genome-wide association studies, which is what’s needed to find genetic risk factors for adult-onset disease.”
• Larger studies. Dr. Wiggs says another restraining factor was an insufficient number of cases and controls. “In the beginning investigators were doing this kind of analysis using really small sample sizes, like 300 cases and 300 controls,” she explains.
“That just wasn’t enough to provide a statistically significant result. It wasn’t until 2009 that we were able to get funding for the GLAUGEN project—the first large-scale genome-wide association study for primary opren-angle glaucoma—that included 1,000 cases and 1,000 controls. But the real progress occurred when we were able to conduct the NEIGHBOR study, which added another 2,500 cases and 2,500 controls. This allowed us to do a genome-wide association study that had some statistical significance for both POAG and a normal-tension glaucoma subset.
|
“Even so, these studies have only found a half-dozen genetic risk factors for POAG, normal-tension glaucoma, pseudoexfoliation glaucoma and angle-closure glaucoma,” she says. “These genes are the tip of the proverbial iceberg; to develop a comprehensive picture of the genetic factors that influence susceptibility to adult-onset disease we have to find a lot more genes. To do that, we need even larger sample sizes. That brings us to the NEIGHBORHOOD study, which is going on right now. More than 20 sites are collaborating on one study; as a result we have about 4,000 cases and 30,000 controls. In addition, we’re contributing data to international studies that also have very large sample sizes. These large studies should finally allow us to find the majority of genetic risk factors that are contributing to adult-onset disease.”
• More sophisticated devices in clinics. “Another factor contributing to new breakthroughs in this area is the availability of clinical measurement devices like swept-source OCT,” notes Dr. Wiggs. “Technologies like this can help define genetically important subgroups of glaucoma patients that have a less-complex genetic underpinning, making the genes in question easier to find.
“For example, in one study we looked at the 10 to 12 percent of glaucoma patients who only have loss of vision in the very center of the visual field, which we believe represents a clinical subtype of glaucoma,” she says. “We’ve already found three genetic risk factors associated with this type of vision loss. The most recent work on this was recently described in the journal Ophthalmology.”1
• Rare variant analysis. “Rare variant analysis allows researchers to identify specific changes in genes that may be responsible for the biological changes under investigation,” says Dr. Wiggs. She notes that the markers that have been studied and used in some screening tests usually do not have a functional, biological effect (although there are exceptions); instead they indicate the presence of a nearby gene or mutation that is having a biological impact. “It wasn’t until the development of the rare variant analysis that we’ve been able to identify the specific changes in the genes that are actually causing the problem,” she says. “We’re in the midst of doing that kind of analysis as part of the NEIGHBOR project.”
Current Developments
“In addition to the really large studies, several things are happening right now that are really exciting,” notes Dr. Wiggs. “One is using modern technologies to identify mutations in families with early-onset glaucoma to help define the diagnosis and inform genetic counseling. Exome-sequencing technology has allowed us to develop test panels that can find the mutations related to early-onset disease in about 90 days; in the past that could have taken a year. So now we can test families with early-onset glaucoma; if they have a mutation in a known gene, we can identify who is at risk and who’s not at risk, which can be a very important piece of information for the family. We can also restrict treatment to the family members who carry the mutations.”
Dr. Wiggs adds that there has been early progress toward gene-targeted therapies. “For example,” she says, “there’s some evidence that by using chemical chaperones, we may be able to treat the underlying molecular mechanism of disease caused by mutations in the myocillin gene.
“All of this sounds complicated, but ultimately, we want to do a very simple thing,” she says. “In the glaucoma clinic we want to be able to sit down with patients and tell them what their risk of developing the disease is, based on their genetic background and environmental exposure history. And of course, we’d love to tailor therapy to those factors that are specific to the patient’s genetic situation. Eventually, we may be able to use gene-based and gene-directed therapies to mitigate or cure the disease.
“We’re definitely reaching a point of critical mass in this research,” she concludes. “That’s what makes it really exciting right now. Until recently, we were missing the right resources to do the experiments. We needed the human genome to be completed; we needed the haploid map to show us how to look at the relevant markers; and we needed the large sample sizes to conduct the analyses. It took a while to reach that point, but now our perseverance is ready to pay off.”
Long-term Drug Delivery
With patient compliance an ongoing problem in glaucoma, long-term delivery of glaucoma medications has become a high priority for many researchers. Several high-tech delivery methods are now in the pipeline.
One approach being developed at the Singapore National Eye Center involves subconjunctival injection of nanoliposomal latanoprost.
A group headed by Tina Wong, MBBS, PhD, FRCOphth, senior consultant ophthalmologist to the Glaucoma Service at Singapore National Eye Centre and head of the Ocular Therapeutics and Drug Delivery Research Group at the Singapore Eye Research Institute, developed the drug formulation, called Lipolat. Nanoparticles in the formulation slowly release the latanoprost over time after injection.
|
"A lot of groups are working on different models [for long-term drug release]. The big challenge is making sure these models work as well in humans as they work in animal models."
–Ramesh S. Ayyala, MD |
“The liposomal latanoprost comes in ready-to-use individual vials that you draw up with a 1-mL insulin syringe,” says Dr. Wong. “With the patient in the clinic we apply topical anesthesia to the superior bulbar conjunctiva using an orange stick soaked in the drug; then the liposomal latanoprost is delivered subconjunctivally via a 27-g. needle. The advantages of delivering the drug in this way include fast and easy administration in the clinical setting and no learning curve for the ophthalmologist.”
The protocol has now been tried in both monkeys and humans, with positive results. In ocular hypertensive monkeys, a single injection lowered IOP for 120 days, with efficacy similar to that of daily drop use. A more recent open-label, pilot study involved six human patients with ocular hypertension or open-angle glaucoma. Unmedicated baseline pressures ranged from 25 to 33 mmHg; each patient received a single injection of 100 µl of Lipolat containing 100 µg of latanoprost.
Patients were followed for three months, including monitoring diurnal IOP in months one and three. All patients had at least a 5-mmHg drop in pressure; three patients (50 percent) had a reduction ≥10 mmHg. Pressure dropped within one hour of receiving the injection and remained reduced throughout the three-month period; no patients showed signs of inflammation or other safety issues. “The data so far show an average IOP lowering of 20 percent from baseline, lasting at least three months,” says Dr. Wong. At this writing, the study had just been accepted for publication in the journal Drug Delivery and Translational Research.
Dr. Wong says they have tried this approach using other glaucoma drugs. “Some work well, others not as well,” she notes. “It depends in part on the structural properties of the drug.” She adds that the group is planning a larger multicenter study next. “Eventually, we hope to develop long-term delivery of other medications using the same platform technology,” she says. “We hope this system will be available for widespread use within three years.”
A Polymer Drug Depot
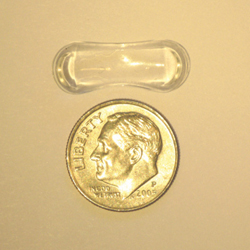
Another system for long-term drug delivery currently under investigation is the Topical Ophthalmic Drug Delivery Device, or TODDD, being developed by Amorphex Therapeutics in Andover, Mass. “TODDD is a soft, elastomeric polymer insert designed to provide sustained drug release,” explains Robert M. Feldman, MD, Richard S. Ruiz MD Distinguished University Professor, and chairman of the Ruiz Department of Ophthalmology and Visual Science at the University of Texas Medical School in Houston. “The insert, which is smaller than a dime, consists of a drug depot carrier, where the drug or drugs are contained in distinct chambers, and a matrix, throughout which the drug can be dispersed. The insert rests on the sclera under the eyelid; it’s shaped so that it will stay in place.”
|
“One TODDD can disperse multiple drugs simultaneously,” he adds. “Drugs incorporated into the TODDD insert thus far include timolol maleate, prostaglandins, pilocarpine, brimonidine, dexamethasone, prednisolone, ciprofloxacin, ibuprofen and lidocaine, demonstrating that the platform has the ability to potentially deliver many agents and treat many diseases.”
Dr. Feldman notes that once inserted, the TODDD provides as much as three months of constant drug delivery, depending on the drug and whether it’s placed in the matrix or the depot carrier. “Patients using this device wouldn’t have to remember to take their drops daily, eliminating daily patient compliance issues,” he points out. “Potential disadvantages would include possible patient intolerance and cost, as well as the possibility that the patient may not know if the device falls out.” At this point, Dr. Feldman says this technology is at least five years from the marketplace.
Slow-release Antifibrotics
Another system under development is the glaucoma slow-release drug delivery system, or GLASS. Unlike most of the other slow-release systems under development, GLASS is currently being investigated as a means of providing a slow, ongoing release of antifibrotic agents to minimize the postoperative fibrosis that impacts the function of glaucoma drainage devices. Ramesh S. Ayyala, MD, FRCS, FRCOphth, professor of ophthalmology and director of the glaucoma service at Tulane University School of Medicine in New Orleans, explains.
“Currently, 50 to 60 percent of glaucoma drainage devices fail within five years,” he says. “When you place a glaucoma drainage device inside the subconjunctival space, most of the fibrosis happens in the first month. Over the next three months encapsulation sets in, leading to the hypertensive phase. The idea behind the GLASS system is that mitigating that initial burst of inflammation during the first month will translate to longer survival for the drainage device, helping to lower IOP.”
Dr. Ayyala explains that his team developed a biodegradable polymer that melts over a period of two to three months in the subconjunctival tissue in the presence of aqueous. “During the process of biodegrading, the drug or drugs are released for up to one month,” he says. “This produces an antifibrotic effect after glaucoma drainage device surgery. So far, after trying many combinations and quantities of drugs, the most effective setup seems to be a combination of 0.1 µm of mitomycin-C released in an initial three-day burst, followed by 30 days of sustained release of 0.9 mg of 5-fluorouracil.”
Dr. Ayyala’s team uses a unique method to cast the polymer so that it can absorb and then elute the drug. “The method is called ‘breath technology,’ ” he says. “Humid air is blown horizontally across the polymer as it’s being cast; the polymer traps the water molecules from the humid air. In 24 hours the water molecules evaporate, leaving behind 10- to 20-µm holes in the polymer. These are the holes through which the drug will elute. You can see them in electron microscopy photographs of the polymer.”
To use both mitomycin-C and 5-fluorouracil, the drugs are loaded in separate layers. “The 5-fluorouracil is loaded first; then mitomycin-C is loaded on top,” he explains. “The result is that the mitomycin gradually comes out in the first 24 to 48 hours to provide the initial burst that kills the fibroblasts that are coming in. The sustained 5-fluorouracil releases slowly over the next 30 days.”
To test the effectiveness of this approach, Dr. Ayyala and colleagues recently conducted a study using two different polymer implants containing drugs, as well as a control implant, all attached to the end plate of Ahmed glaucoma valves implanted into rabbit eyes. One device, made of a polyhydroxyethylmethacrylate-based nonbiodegradable polymer, released mitomycin-C for about three weeks. The second device, made of their biodegradable poly (lactic-co-glycolic acid) polymer, released an initial three-day burst of mitomycin-C followed by 30 days of sustained-release 5-fluorouracil. Three months post-surgery the rabbits were sacrificed to evaluate the bleb wall thickness around the end plate.
At three months the bleb roof thickness of the control group was 544 ±170 µm; the nonbiodegradable polymer implant group was 373 ±143 µm (p=0.006); and the biodegradable dual-drug implant group was 316 ±55 µm (p<0.001). The polymer in the latter eyes had completely disappeared, as expected, and there were no cases of infection or avascular cystic bleb formation in any eyes.
Dr. Ayyala says that their work so far with animal models suggests that the fibrosis reaction and encapsulation is decreased by more than 50 percent when the GLASS system is employed. “We’ve seen as much as a 70-percent reduction in fibrosis at the end of three months in the rabbits,” he says. “The other important thing about the system is that we’re using less than 70 percent of the amount of mitomycin-C that many surgeons currently inject into human eyes at the time of trabeculectomy or Express shunt surgery.”
Dr. Ayyala also notes that they haven’t seen any side effects at all in the animal model. “We’ve used GLASS in about 150 rabbits so far,” he says. “Not one rabbit has had an eroded conjunctiva or any cystic bleb formation. The blebs are nice and the encapsulation is there, but it’s really thin. If this holds true in humans, then we’ll have a very useful adjunct to glaucoma shunt surgery.”
Taking It One Step Further
Dr. Ayyala observes that the hypertensive phase usually happens between postop day 30 and 90. “Normally we use an aqueous suppressant like Cosopt to reduce any IOP spike during the hypertensive phase,” he says. “Now we’re considering the possibility of having a third layer of drug behind the 5-fluorouracil, perhaps an aqueous suppressant such as timolol. The timolol would start to come out at the end of one month, after the 5-fluorouracil is completely released. If this strategy works, it will decrease IOP during the hypertensive phase. However, this possibility is still in the early stages.
“Once the pilot studies of the current device are initiated, we’ll also be trying out a variation on the current model that we think will help with Express shunts and trabeculectomies,” says Dr. Ayyala. “All you’ll need to do is place the device in the area of the operation and close the flap. The polymer will release the drug very slowly over a period of one month. We’re getting ready to do animal-model studies on that. In addition, we’re in the early stages of developing a polymer device for delivering glaucoma medications that can be applied just like a drop; when applied into the inferior fornix it will latch onto the conjunctiva and release the drug over a period of three months.
“In fact, a lot of groups are working on different models like these,” he says. “The big challenge is making sure these models work as well in humans as they work in animal models.” (To read about two more slow-release devices in the works, see Technology Update in the January 2014 issue of Review.)
REVIEW
Dr. Ayyala owns a patent on the GLASS system and has formed a company to manufacture the device. Dr. Feldman has no financial ties to the technologies discussed. Dr. Wong is co-owner of the Lipolat patent.
1. Loomis SJ, Kang JH et al. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. Ophthalmology 2014;121:2:508-16. doi: 10.1016/j.ophtha.2013.09.012. Epub 2013 Oct 25.