Refractive surgery is clearly a success story. To date, about 40 million LASIK procedures have been performed globally, and the success rate has been reported to be around 97 percent, with patient satisfaction rates around 95 percent. However, most refractive surgery patients are myopes, and myopia is a risk factor for glaucoma. This means that many patients coming in for refractive surgery are either glaucoma suspects or will develop glaucoma later in life. Couple this with the current myopia epidemic and we have a recipe for glaucoma problems both now and in the future.
In this article I’ll share some tips for refractive surgeons that should reduce the likelihood of glaucoma-related problems developing in patients considering refractive surgery. Then, I’ll offer some strategies glaucoma specialists can employ to manage eyes post-refractive surgery.
The Scope of the Problem
From the perspective of the refractive surgeon, the potential for many myopic individuals to develop glaucoma later in life might not seem like a big problem, since those seeking refractive surgery tend to be young and healthy. However, the reality is that we’re in the midst of a myopia epidemic. In 2010 about 28 percent of the world’s population was myopic, but this is projected to increase to 50 percent by the year 2050. In East Asia, myopia rates are reaching almost 90 percent.
This is concerning from a glaucoma standpoint, because many well-designed, large population-based studies have reported a two-to-four-fold increased prevalence of glaucoma among myopic subjects.1-4 That correlation is even higher with moderate to high levels of myopia. So as more and more myopic individuals are seeking refractive surgery, it’s becoming increasingly important to screen these patients with glaucoma in mind, and to be aware of the possible interactions between refractive surgery and glaucoma during and after a procedure.
The other side of this story is that an increasing number of patients requiring treatment for glaucoma have had refractive surgery in the past. This adds up to a number of special challenges for a doctor managing the patient’s glaucoma. However, those challenges can be reduced if refractive surgeons take some extra steps before the procedure.
The Refractive Surgery Options
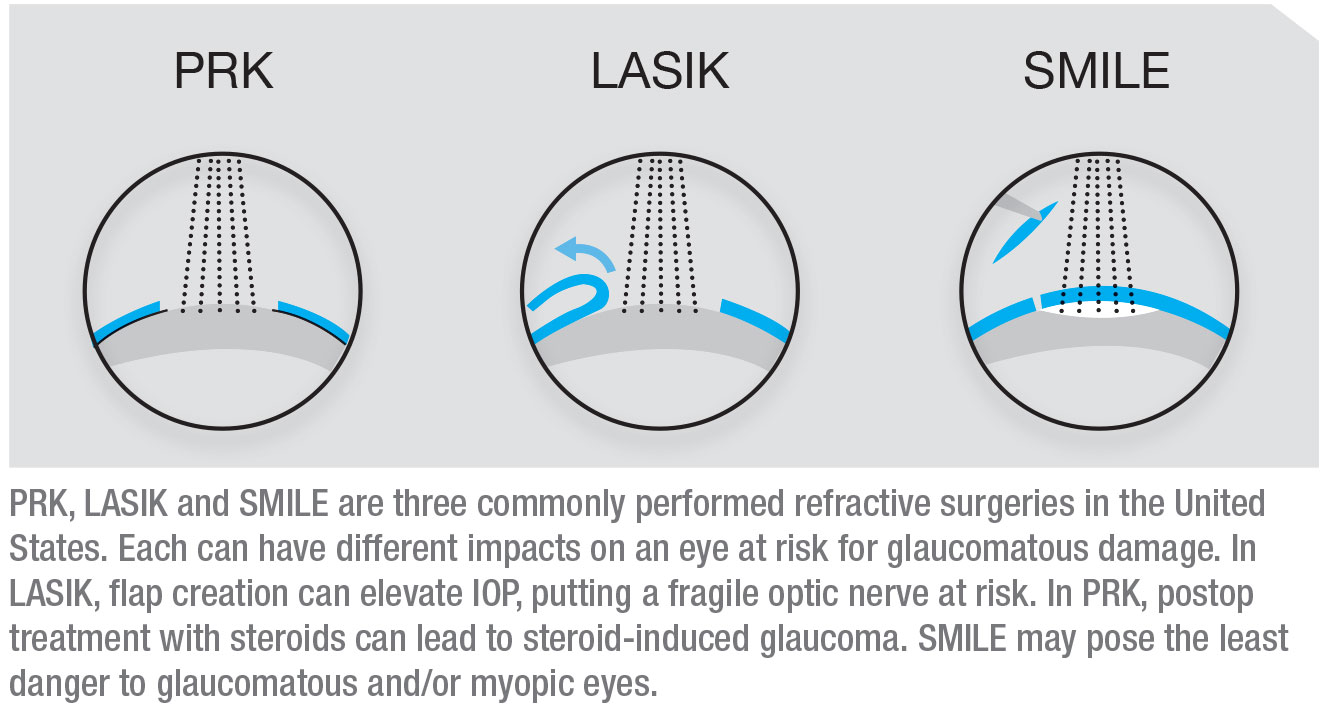
Three commonly performed refractive surgeries in the United States are LASIK, PRK and SMILE. Each can have different potential impacts on an eye at risk for glaucomatous damage, and all three can have an impact on the eye in terms of future management of glaucoma. Both refractive surgeons and glaucoma specialists need to be aware of these issues.
When LASIK is performed, a flap is created using either a microkeratome or a femtosecond laser; then the stroma is ablated to create the refractive correction. The advantage of LASIK is that the visual recovery is much faster than the recovery after PRK, but LASIK patients are prone to flap-related problems. In terms of glaucoma, one of the main issues is that when the flap is created, the pressure in the eye may be elevated to a very high level that can potentially further damage a compromised optic nerve.
When performing PRK, the epithelium is removed mechanically or chemically; this is followed by surface ablation using an excimer laser. No flap is created, limiting complications related to increased intraoperative IOP. The downside of PRK is that removing the epithelium causes the visual recovery to take much longer than recovery from LASIK, and the recovery usually requires treatment with steroids. That’s a concern for a glaucoma suspect—and even a healthy myope—because these individuals are at risk of getting steroid-induced glaucoma.
The most recent addition to the refractive surgery armamentarium is SMILE, a sort of minimally invasive version of LASIK. A femtosecond laser creates an internal lenticule of tissue in the cornea which is then removed through a small incision (about 2 mm), resulting in a refractive correction. There’s no flap, and no excimer laser is involved. Because of the small incision, the visual recovery is even faster than following LASIK. Among the three procedures, SMILE may pose the least danger to myopic and/or glaucomatous eyes.
Preop Considerations
 |
To minimize potential issues when performing refractive surgery on myopes (whether or not they qualify as glaucoma suspects or patients) and make it easier to manage glaucoma further down the line should it develop, refractive surgeons can do the following:
• Actively inquire about a family history of glaucoma. Young myopes coming for refractive surgery aren’t thinking about family members who may have glaucoma. They’re often young and healthy, in their 20s. Therefore, it’s important for the physician to establish a good baseline with ancillary testing and to recommend close monitoring.
• Make sure these patients under-stand that they have a higher-than-average risk of glaucoma in the future. Be sure to educate the patient about the association between myopia and glaucoma. They can’t take steps to protect themselves if they don’t know this is an issue.
• If your patient has glaucoma, consider performing SMILE or
PRK instead of LASIK. PRK and SMILE may be better options be-cause they don’t require creating a flap, thus avoiding IOP elevation that could potentially affect an already compromised optic nerve during flap creation.
• Do a preop OCT glaucoma scan. In addition to potentially revealing information relevant to the patient’s suitability for undergoing refractive surgery, this will provide a baseline for comparison if the patient develops glaucoma in the future.
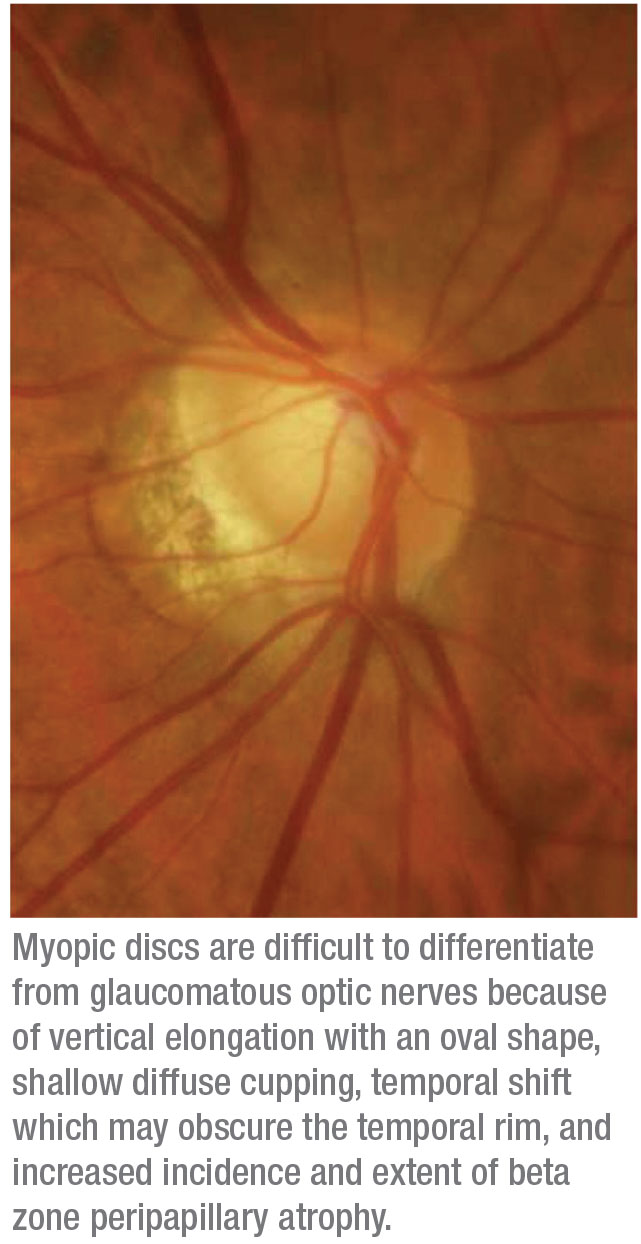
• Do preop perimetry, especially in myopes. If these patients do develop glaucoma, their visual fields can be challenging to interpret because many myopic patients display visual field defects even if they don’t have glaucoma. A large percentage of myopic individuals, almost 80 percent in one study, can develop visual field defects without having glaucoma. These defects often resemble glaucomatous defects—nasal steps, superior or inferior arcuate defects and paracentral defects.5 (See example, left.) As a result, it’s hard to distinguish which visual field abnormalities are due to myopia and which are due to new-onset glaucoma. Having a pre-refractive-surgery baseline visual field may help identify the new defects related to glaucoma.
• Include gonioscopy in your preop protocol, especially with hyperopes. Gonioscopy is routinely performed by glaucoma specialists in all patients to determine whether the angle in the eye is open or closed, and to tailor an appropriate intervention. This is typically not done by our refractive colleagues. Although I’d recommend doing gonioscopy on every pre-refractive-surgery patient, it’s most important in hyperopic patients, because cases of acute angle-closure glaucoma have been reported after hyperopic LASIK.6,7 Hyperopic patients have smaller eyes, a more congested anterior segment and narrow angles that make them susceptible to acute angle-closure glaucoma.
The other important diagnosis that can be determined by gonioscopy is pigment dispersion syndrome. At times the clinical signs of PDS may not be readily apparent on the slit lamp exam. PDS is common in myopes and is a risk factor for developing pigmentary glaucoma. Because of co-existing myopia, eyes with PDS are also at high risk of steroid-induced glaucoma.8 Therefore, these patients should be closely monitored.
• Include disc photos in your preop protocol. Today, getting OCTs before refractive surgery is common. However disc photos are still very valuable, especially when it comes to myopic individuals. The optic nerve appearance in these patients can be difficult to interpret, because in a myopic eye it sometimes has an abnormal appearance that’s similar to an eye with glaucoma. Having a preop baseline photo is extremely helpful for monitoring these patients over time.
I’ve cared for many patients with IOP measurements in the low to normal range resulting from stromal ablation after refractive surgery. In these cases, careful examination of the optic nerves and comparison to baseline optic disc photos can be extremely helpful. The stereo disc photos give us an objective way to follow these eyes. It’s certainly better than going by a written description; these may differ because of inter-observer variability.
• Consider measuring IOP with more than one instrument. After refractive surgery, GAT is likely to underestimate IOP, making it difficult to both diagnose and monitor glaucoma. It’s important to measure IOP with different instruments that
are less likely to be affected by stromal ablation, such as the Ocular Response Analyzer or dynamic contour tonometry, for more accurate measurements.
 |
Intraoperative IOP
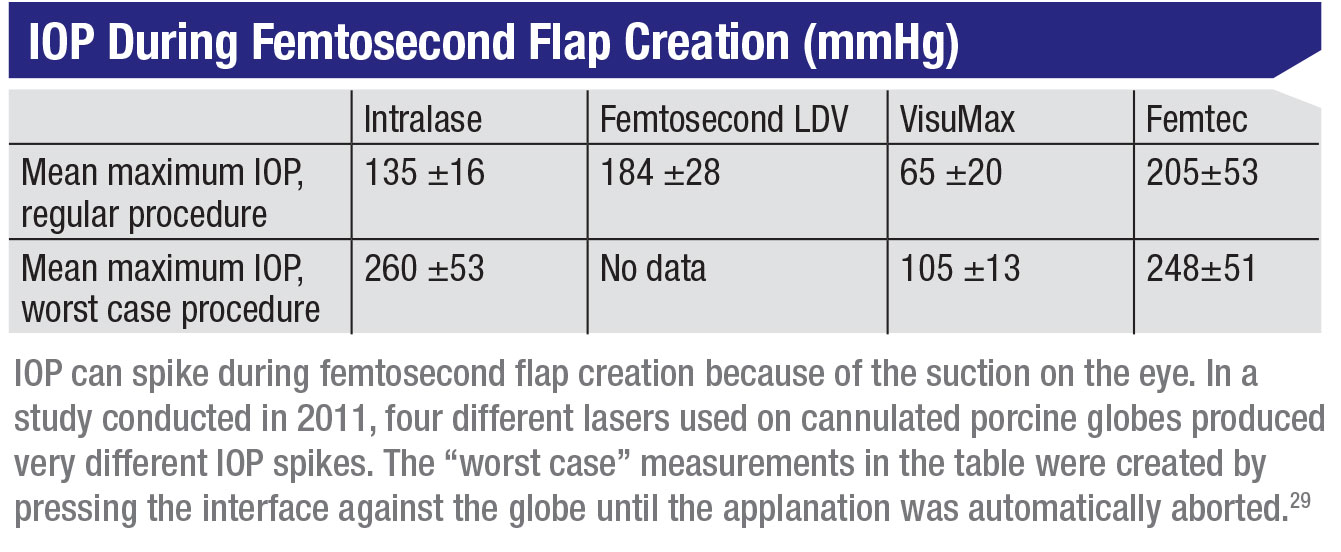
Today about 70 percent of LASIK flaps are being created us-ing a femtosecond laser, but both femtosecond laser flaps and microkeratome flaps elevate the IOP during flap creation, putting a fragile or damaged optic nerve at risk. Cases of optic neuropathy and visual field loss have been reported with the use of microkeratomes, although the underlying mechanisms remain unclear. It could be that ischemia results from the extreme elevation of IOP during the flap creation, or that it’s secondary to direct trauma to the optic nerve head. IOP elevation that occurs with different femtosecond laser platforms can range anywhere from 65 to 260 mmHg, as has been demonstrated in porcine eyes. (See table.)
The point is that no matter how the flap is created, extreme pressure elevation can potentially occur. The elevation is transient, but reports of problems have surfaced. Maybe the problems occurred because the patients were either glaucoma suspects or had undiagnosed glaucoma. Therefore, careful screening is warranted.
Postop Considerations
Following refractive surgery, a number of glaucoma-related problems can arise:
• After PRK, watch for steroid-induced glaucoma. The biggest glaucoma-related issue in the post-operative period is associated with the use of steroids, primarily following PRK. PRK patients are often put on steroids, making them more likely to develop a steroid response.
Of course, not every patient with a higher-than-average risk for glaucoma is going to develop steroid-induced glaucoma. It depends on the dose of the medication; the chemical structure of the medication; the frequency and route of delivery, which will usually be topical in these cases; and of course the duration of the treatment. It also depends on patient-related variables such as having glaucoma, being a glaucoma suspect, having a family history of glaucoma and being myopic. Even a young, healthy patient who is a myope, or who has a family history of glaucoma is at risk of developing steroid-induced glaucoma. That means refractive surgeons need to pay attention to this.
• After LASIK, watch for pressure-induced stromal keratitis. Post-LASIK there are other concerns. One is the condition called pressure-induced stromal keratitis, or PISK. PISK causes elevated IOP and diffuse interlamellar inflammatory haze that covers most of the flap diameter. One of the issues associated with PISK is that it’s easily confused with diffuse lamellar keratitis, or DLK. It’s important to distinguish between them, because they’re treated in opposite ways. DLK is treated with steroids, while PISK is caused by steroids. If the IOP is high because of PISK and you’re just treating with IOP-lowering medications, that’s not going to help. You have to discontinue the steroids in order to achieve both resolution of the keratitis and normalization of IOP. 9-11
There are two ways to distinguish between DLK and PISK: timing and response to treatment. DLK tends to happen soon after the refractive surgery, while PISK usually happens beyond the first postop week. The other way to tell them apart is by the response to your treatment, as mentioned above. If you mistake PISK for DLK and increase steroids, the problem will get worse.
• Remember that a postop loose LASIK flap or interface cyst will falsely lower the IOP measurement. Other postop LASIK problems that relate to glaucoma include loose flaps and interface cysts, either of which will affect the accuracy of IOP measurement when monitoring for issues such as postop steroid-induced glaucoma. When measuring IOP in the presence of a loose flap or an interface cyst, the force required to applanate the overlying flap is dampened because of the loose flap or the fluid under the cyst, causing an artificially low reading. So, you may think the pressure is normal or low when it’s actually elevated. Treatment of either condition involves tapering of steroids and the use of IOP-lowering medications.12-16
• Monitor the patient regularly in the years after surgery. Myopia is a risk factor for glaucoma; there-fore, even an uneventful surgery and postop period may eventually be followed by glaucoma. All myopic refractive surgery patients should be monitored regularly.
IOP After Refractive Surgery
As glaucoma specialists, we rely on the Goldmann applanation tonometer as our gold standard for IOP measurements. But GAT is limited in this particular population, because after refractive surgery and stromal ablation, corneal thickness varies. Goldmann designed his tonometer to be most accurate when measuring a cornea with a central corneal thickness of 520 µm. Using GAT at that thickness, the opposing forces of surface tension and corneal rigidity balance each other out and can be ignored. But after a cornea has undergone refractive surgery, that magic number is no longer useful. As a result, most studies in the literature indicate that GAT underestimates IOP after refractive surgery.
That’s very important, because if pressure is being measured low, we can mistakenly believe a patient is normal when he isn’t. It’s critical in such cases to look at other parameters during our examination and testing to make sure we’re not missing glaucoma in these individuals—and we have to choose our method of IOP measurement carefully.
Important things to keep in mind include:
• Be aware that glaucoma patients may not mention past refractive surgery. Patients who had refractive surgery 20 years ago some-times think it was just some minor procedure that no longer matters. They won’t volunteer the information unless you actively seek it. I’ve learned to ask patients specifically if they were very nearsighted early in life and whether they’ve had LASIK or PRK.
• Obtain any pre-refractive-surgery data that you can. Although we don’t have any algorithms for converting baseline IOP data to current equivalents, it’s helpful to know how high the patient’s myopia was and/or how much ablation was done. This information can help approximate the real IOP.
• The change in IOP correlates with the ablation depth. The more ablation was done, the more the CCT is reduced and the greater the impact on measured IOP.
• The decrease in measured IOP is more pronounced in myopic patients than hyperopic patients, for both LASIK and PRK. Although I don’t know for certain why this is true, it could have something to do with the elongated shape of the myopic eye, which can be associated with a thinner cornea. Another possible explanation is that a myopic ablation is usually focused on the center of vision—where GAT is typically measured—while hyperopic ablation tends to focus pericentrally.
• It’s important to measure IOP with more than one instrument. Since GAT is likely to underestimate IOP, we should be measuring IOP using different instruments. The more information you have, the better. Currently, several tonometers are available to us, including the tonopen, pneumotonometer, ORA and DCT.
Some of these can be used to measure IOP at the periphery of the cornea after refractive surgery, instead of in the center. However, it’s important to keep in mind that the peripheral cornea tends to be thinner than the central cornea, even if the refractive surgery hasn’t altered the periphery. That means that peripheral IOP measurements will always tend to be a little lower than central measurements. Again, this kind of strategy is most helpful if such a measurement was taken prior to the refractive surgery, allowing a fair comparison.
 |
Two tonometers that seem to be better at measuring post-refractive-surgery IOP are the ORA and the DCT. Their measurements have been shown to be less affected by stromal ablation.17-21 For example, the DCT consists of an electronic strain gauge that’s embedded in a contoured plastic tip. When the DCT measures IOP, it doesn’t applanate the corneal tissue, making the measurement in-dependent of corneal properties. The ORA measures corneal hysteresis, a measure of the resiliency or shock-absorbency of the cornea, in addition to IOP, using a rapid air impulse to apply force to the cornea. It then adjusts the IOP measurement to take the corneal hysteresis into account. Studies suggest that this results in an accurate IOP measurement despite corneal alterations from refractive surgery. (For more on the ORA, see the Point/Counterpoint feature.)
• Remember that structural imaging (including OCT) seems to be unaffected by refractive surgery. Most studies have demonstrated that there are no changes in retinal nerve fiber layer thickness measurement after refractive surgery.22-26 These findings have been confirmed in microkeratome-assisted LASIK, femtosecond assisted LASIK and SMILE. This means we don’t have to develop a new baseline for imaging in these patients. That’s good, because imaging has become a routine part of glaucoma evaluation and often provides evidence of structural damage before functional loss occurs.
However, it’s also important to remember that most refractive surgery patients are myopes, and myopes tend to have artifacts on OCT simply because of their eye structure. A comparison to a baseline scan can still be revealing, though, since changes can be observed, even in the presence of artifacts.
|
• Be aware that visual field changes can result from issues with the way the refractive surgery was performed. At least one study conducted back in 2005 reported that visual field changes occurred after LASIK in the absence of optic nerve or retinal nerve fiber layer damage.27,28 The authors concluded that this was due to under-ablation of the paracentral cornea, causing irregular optical zones. Obviously, the nature of LASIK surgery and the algorithms used today are far better than those in use 15 years ago, but the possibility that the refractive surgery may be affecting the visual field is worth keeping in mind, since some of your glaucoma patients may have had refractive surgery that long ago.
Making the Best of It
There’s no question that refractive surgery and glaucoma each affect the other. Myopia is a risk factor for glaucoma and a major driver of individuals seeking refractive surgery; therefore, overlap is inevitable. Given this reality, both refractive surgeons and glaucoma specialists can help patients by acknowledging this over-lap and taking steps to mitigate any damage.
Refractive surgeons can help these patients by doing three things:
1) Assist the doctors who may treat glaucoma in these patients later on by doing a few extra things before surgery: proactively inquire about any family history of glaucoma; educate myopic patients about their increased risk of glaucoma and advise them to have regular follow-ups; perform a comprehensive exam, including gonioscopy and IOP measurements using different devices; and obtain baseline ancillary tests, including disc photos, imaging and visual fields.
2) Do what you can to avoid worsening any existing problem during the surgery, by avoiding flap creation if possible, or using a flap creation system that doesn’t raise IOP too excessively, to minimize damage to an already compromised nerve.
3) Be alert for the presence of steroid-induced glaucoma, pressure-induced stromal keratitis, an interface cyst or a loose flap during the postop period.
Glaucoma specialists can help these patients by:
1) proactively inquiring about refractive surgery in the past;
2) obtaining any pre-refractive-surgery data that may be available;
3) measuring IOP in ways that are the least likely to be altered by the earlier refractive surgery; and
4) paying attention to other para-meters of glaucoma evaluation in the presence of inaccurate IOP measurements. REVIEW
Dr. Salim is a professor of ophthalmology, vice chair of clinical and academic affairs, and director of the Glaucoma Service at Tufts University School of Medicine. She is a consultant and speaker for Aerie Pharmaceuticals and a speaker for Bausch + Lomb.
1. Jiang X, Torres M, Varma R; Los Angeles Latino Eye Study Group. Variation in intraocular pressure and the risk of developing open-angle glaucoma: The Los Angeles Latino Eye Study. Am J Ophthalmol 2018;188:51-59.
2. Shen SY, Wong TY, Foster PJ, et al. The prevalence and types of glaucoma in Malay people: The Singapore Malay Eye Study. Invest Ophthalmol Vis Sci 2008;49:9:3846-51.
3. Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology 1992;99:10:1499-504.
4. Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: The Blue Mountains Eye Study. Ophthalmology 1999;106:10:2010-5.
5. Kumar RS, Baskaran M, Singh K, Aung T.J Clinical characterization of young Chinese myopes with optic nerve and visual field changes resembling glaucoma. Glaucoma 2012;21:5:281-6.
6. Paciuc M, Velasco CF, Naranjo R. Acute angle-closure glaucoma after hyperopic laser in situ keratomileusis. Cataract Refract Surg 2000;26:4:620-3.
7. Osman EA, Alsaleh AA, et al. Bilateral acute angle closure glaucoma after hyperopic LASIK correction. Saudi J Ophthalmol 2009;23:3-4:215-7.
8. Jabbur NS, Tuli S, et al. Outcomes of laser in situ keratomileusis in patients with pigment dispersion syndrome. J Cataract Refract Surg 2004;30:1:110-4.
9. Belin MW, Hannush SB, et al. Elevated intraocular pressure-induced interlamellar stromal keratitis. Ophthalmology 2002;109:10:1929-33.
10. Davidson RS, Brandt JD, Mannis MJ. Intraocular pressure-induced interlamellar keratitis after LASIK surgery. J Glaucoma 2003;12:1:23-6.
11. Cabral-Macias J, García-De la Rosa G, et al. Pressure-induced stromal keratopathy after laser in situ keratomileusis: Acute and late-onset presentations. Cataract Refract Surg 2018;44:10:1284-1290.
12. Lyle WA, Jin GJ. Interface fluid associated with diffuse lamellar keratitis and epithelial ingrowth after laser in situ keratomileusis. J Cataract Refract Surg 1999;25:7:1009-12.
13. Handzel DM, Stanzel BV, Briesen S. [Complication cascade after hyperopic LASIK]. Ophthalmologe 2011;108:7:665-8.
14. Bamashmus MA, Saleh MF. Post-LASIK interface fluid syndrome caused by steroid drops. Saudi J Ophthalmol 2013;27:2:125-8.
15. Shoji N, Ishida A, et al. Interface fluid syndrome induced by uncontrolled intraocular pressure without triggering factors after LASIK in a glaucoma patient: A case report. Medicine (Baltimore) 2015;94:39:e1609.
16. Kim CY, Jung YH, et al. Delayed-onset interface fluid syndrome after LASIK following phacotrabeculectomy. MC Ophthalmol 2019;19:1:74.
17. Yao WM, Crossan AS. An update on postrefractive surgery intraocular pressure determination. Curr Opin Ophthalmol 2014;25:4:258-63.
18. Hosny M, Aboalazayem F, et al. Comparison of different intraocular pressure measurement techniques in normal eyes and post small incision lenticule extraction. Clin Ophthalmol 2017;11:1309-1314.
19. Schallhorn JM, Schallhorn SC, Ou Y. Factors that influence intraocular pressure changes after myopic and hyperopic LASIK and photorefractive keratectomy: A large population study. Ophthalmology 2015;122:3:471-9.
20. Ajazaj V, Kaçaniku G, et al. Intraocular pressure after corneal refractive surgery. Med Arch 2018;72:5:341-343.
21. Zhang H, Z, et al. Comparison of intraocular pressure mea-sured by ocular response analyzer and Goldmann applanation tonometer after corneal refractive surgery: A systematic review and meta-analysis. MC Ophthalmol 2020;20:1:23.
22. Aristeidou AP, Labiris G, et al. Comparison between Pascal dynamic contour tonometer and Goldmann applanation tonometer after different types of refractive surgery. Graefes Arch Clin Exp Ophthalmol 2011;249:5:767-73.
23. Sharma N, Sony P, et al. Effect of laser in situ keratomileusis and laser-assisted subepithelial keratectomy on retinal nerve fiber layer thickness. J Cataract Refract Surg 2006;32:3:446-50.
24. Dementyev DD, Kourenkov VV, et al. Retinal nerve fiber layer changes after LASIK evaluated with optical coherence tomography. J Refract Surg 2005;21(5 Suppl):S623-7.
25. Zhang J, Zhou Y, et al. Effect of suction on macular and retinal nerve fiber layer thickness during femtosecond lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis. J Cataract Refract Surg 2014;40:12:1994-2001.
26. Katsanos A, Arranz-Marquez E, et al. Retinal nerve fiber layer thickness after laser-assisted subepithelial keratomileusis and femtosecond LASIK: A prospective observational cohort study. Clin Ophthalmol. 2018 Jul 4;12:1213-1218.
27. Kumar RS, Baskaran M, et al. Clinical characterization of young Chinese myopes with optic nerve and visual field changes resembling glaucoma. Glaucoma 2012;21:5:281-6.
28. Brown SM, Bradley JC, et al. Visual field changes after laser in situ keratomileusis. Cataract Refract Surg 2005;31:4:687-93.
29. Vetter JM, Holzer MP, et al. Intraocular pressure during corneal flap preparation: Comparison among four femtosecond lasers in Porcine eyes. J Refrac Surg 2011;27:6:427-33.