Formerly defined in terms of elevated intraocular pressure, glaucoma is better considered a progressive optic neuropathy representing the final common pathway of a number of disorders. Elevated pressure is the most important risk factor for damage, but certainly not the only one. Many risk factors remain poorly understood, and many remain to be discovered.
In recent years, evidence has mounted that many glaucomas are not isolated diseases, but are associated with systemic conditions. These systemic disorders are often considered to be risk factors for progression of glaucomatous damage, or non-pressure-dependent risk factors, but they are also distinct disorders themselves. Since the best treatment for any disease addresses the root cause rather than just relieving symptoms, it makes sense for us to explore these connections. The result could be not only a better understanding of glaucoma, but more effective treatments that might also benefit the patient's overall health and well-being.
Early Clues
My interest in the connections between glaucoma and systemic conditions was sparked at the end of my fellowship. We were already well-aware that developmental glaucomas, such as those occurring in patients with aniridia, Axenfeld-Rieger syndrome, and Sturge-Weber syndrome, are associated with systemic findings. We also knew that uveitis and, concomitantly, uveitic glaucoma, could occur as a component of both infectious and inflammatory systemic conditions.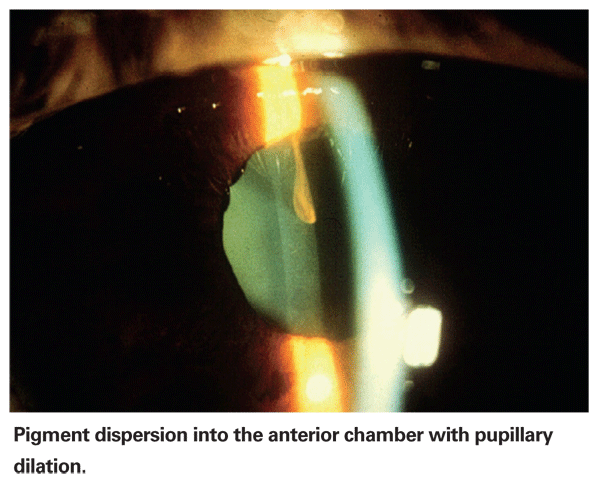
Around that time I had a patient with an intraocular pressure of 48 mmHg who had already undergone two filtering procedures that had failed. He was scheduled for implantation of a Krupin-Denver valve, but the endocrinologist who was seeing him for medical clearance noted that the patient had all the clinical signs of hypothyroidism. He said, "Give me a couple of days before you operate on him; let me put him on thyroxine." To our surprise, within two days, the patient's IOP had dropped to 16 mmHg.1 We initiated a study of newly diagnosed patients with primary hypothydroidism and found that about 40 percent of them had elevated IOP.
In the years since then, accumulating evidence in the literature, along with my own experience in the clinic, has convinced me that many glaucomas may be aspects of systemic conditions rather than isolated ocular disease.
The Pigment Dispersion Connection
During the 1980s, I became interested in pigment dispersion syndrome. I noted that patients presenting with PDS seemed to have a distinct personality type; they tended to be perfectionistic, goal-oriented, obsessive/compulsive and hypomanic. In fact, I often said that PDS seemed to be an occupational disease of lawyers, surgeons and computer analysts. If I walked into an exam room to see a new, younger patient for the first time and he was gripping the edges of the chair, I would make a tentative diagnosis of PDS. (Most of these patients, incidentally, are men.) My residents brought me a PDS patient once, claiming he was the exception to the rule; he was a toll-taker on the Tri-Boro bridge. As it turned out, he couldn't stop talking, telling me how he got twice as many cars through the bridge at rush hour as anybody else! Bernard Becker, MD, and Harold Scheie, MD, also noted in passing that patients with PDS tended to be "tense" or "high-strung."2,3
I've also observed, again anecdotally, what appears to be a greater than average incidence of cardiovascular disease among PDS patients, accompanied by an above-average number of angioplasties and bypass operations. (I haven't yet reviewed our data to determine whether this observation is supported by statistically significant numbers.) Some evidence also suggests that PDS responds to epinephrine compounds better than any other glaucoma, although this has not been formally studied either, but again has been mentioned in passing.2,3 On the basis of this and other findings, I regard PDS as a disorder associated with or characterized by adrenergic hypersensitivity. I have hypothesized that the gene is in the tyrosinase pathway, perhaps involving dopamine or serotonin.
Exfoliation: System-Wide Symptoms
Exfoliation syndrome is the most common identifiable cause of open-angle glaucoma worldwide, comprising the majority of glaucoma in some countries. In 1992, two reports appeared, each describing autopsy findings in a single patient with XFS;4,5 both found exfoliation material throughout the body, primarily in the connective tissue in organs such as heart, cerebral meninges, lung and kidney. The following year, an association was noted between XFS and transient ischemic attacks.6 There has since been a steadily increasing number of reports associating XFS with cardiovascular disorders; not just abnormalities of ocular blood flow, but middle meningeal artery dysfunction, silent myocardial ischemia and carotid artery stiffness.7 XFS has also been associated with Alzheimer's disease and hearing loss.8-11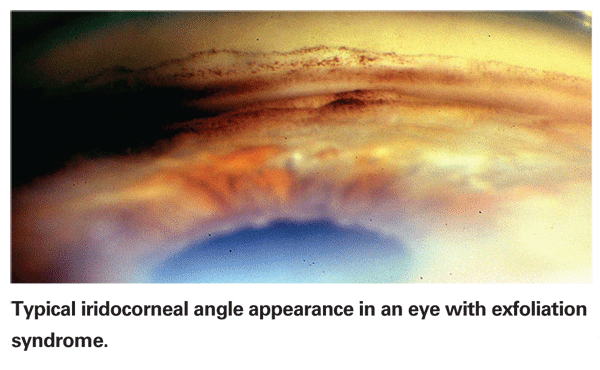
The hearing loss connection is interesting because the cells in the ear that produce and drain the endolymph are structurally analogous to the cells in the eye that produce and drain the aqueous humor. Similar parallels are found in the brain; the choroid plexus and the arachnoid villi are structurally analogous to the ciliary epithelium and the trabecular meshwork. For this reason, investigating the condition of the brain in patients with exfoliation syndrome could reveal important information. Currently, however, this area remains unexplored.
Other related issues have also arisen:
• Fibrillin. It appears likely that XFS is a conformational disorder of fibrillin. Professor Ursula Schlötzer-Schrehardt has found abnormalities in latent transforming growth factor-beta binding protein, which binds fibrillin to the extracellular matrix.12 The extracellular matrix and exfoliation material share many common components.
• The LOXL1 gene. Last year, new published data found another powerful connection between XFS and systemic conditions. Two single nucleotide polymorphisms were discovered in the lysyl oxidase-like 1 gene (LOXL1); these mutations confer susceptibility to exfoliative glaucoma, primarily through exfoliation syndrome.13 (Not everyone with these polymorphisms develops XFS, but 99 percent of those with XFS in several populations had the polymorphisms.14-17) This family of genes is involved in the formation and maintenance of elastic tissue, inducing cross-linking in collagen and elastin molecules, thereby helping to maintain both the extracellular matrix and the walls of blood vessels throughout the body.18
This gene could explain the connection between XFS in the eye and its association with an increasing number of systemic disorders, many of which affect the cardiovascular system and ocular perfusion. For example, one of the polymorphisms associated with XFS is also associated with subacute carotid artery dissection, which is the most common cause of stroke in people under age 55. Other conditions have also developed in mice genetically engineered to be missing the LOXL1 gene, including pelvic floor dysfunction in females and varicose veins.19,20 (So far, no one has conducted a study to see whether human XFS patients suffer from an unusual number of dropped bladders or varicose veins.)
This connection raises a host of questions: Do people manifest disease because of exfoliation material deposited throughout their bodies, or is the exfoliation material just a manifestation of the disease as an ongoing process? Is the LOXL1 gene involved in hearing loss? At this point, we don't know. Hopefully the recent discoveries will inspire a burst of activity in this area.
• Blood levels. About 60 percent of XFS patients have elevated homocysteine levels; the mean homocysteine level in these patients was significantly higher than that in normal tension glaucoma patients or controls, as was the proportion of XFS patients with elevated homocysteine.21-27 (Homocysteine levels are also elevated in patients with Alzheimer's, myocardial infarction, stroke and vascular disease, and there's a connection between homocysteine and the LOXL1 gene.)28-32 In addition, there is a decrease in systemic levels of B6, B12 and folate in patients with XFS.18,21
Clearly these factors reflect systemic conditions and are likely to have systemic consequences.
The Pressure Question
More than 30 years ago, debate raged about whether glaucomatous damage was caused by mechanical pressure or vascular ischemia. Today it's clear that damage can occur in the presence or absence of elevated IOP, leading to the conclusion that glaucoma can be caused by mechanical damage (IOP-related), ischemia (IOP-independent damage) and by other mechanisms, such as increased susceptibility of retinal ganglion cells to insult (another facet of IOP-independent damage). Damage caused by elevated IOP may be the result of a local problem, such as angle closure or XFS, although even in these cases there may be a systemic connection, as we've noted for XFS. Damage caused in the absence of elevated IOP—usually categorized as normal-tension glaucoma—is especially likely to be the result of a systemic problem associated with other signs and symptoms elsewhere in the body.
The presence of more than one avenue for damage is supported by the existence of overlap syndromes. In these cases a patient may have, for example, relatively high IOP, but go for years without developing any sign of glaucoma. Then abruptly something changes, such as the appearance of atrial fibrillation; at that point glaucomatous damage begins to occur.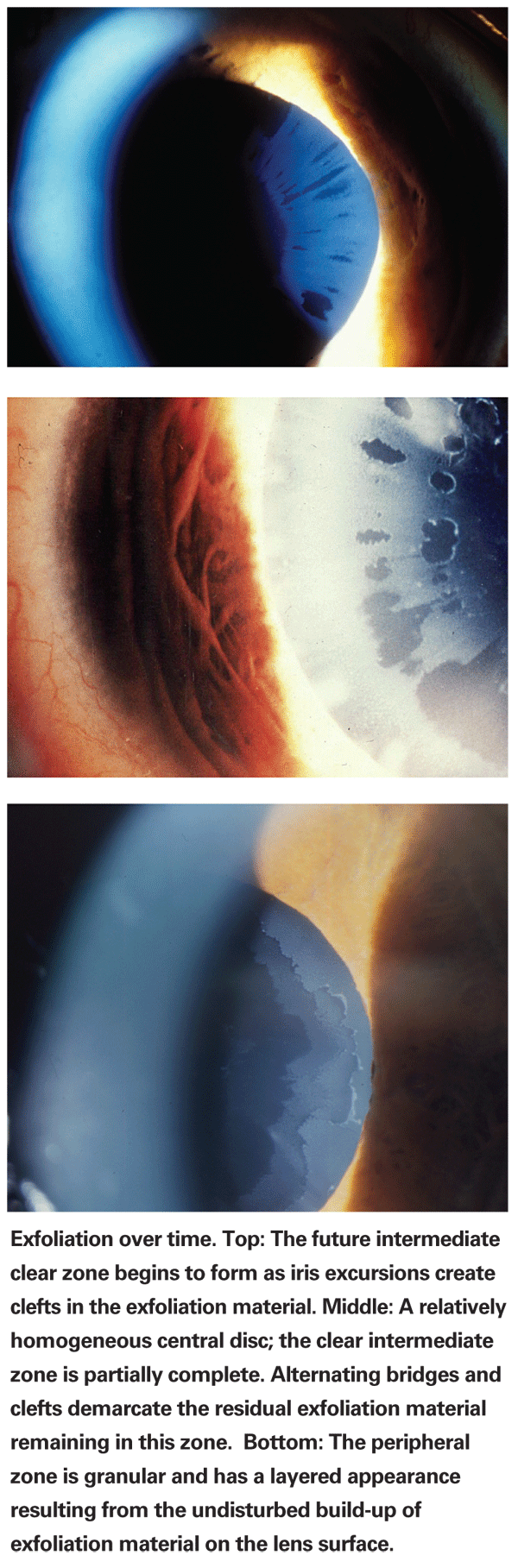
The importance of acknowledging multiple causes for glaucomatous optic neuropathy is that it makes it clear that we need to be looking for avenues of causality—and ways to treat glaucoma—outside the eye as well as inside.
Finding Alternative Therapies
Directed therapy—i.e., specific treatments designed to interfere with the mechanisms of specific diseases—is conceptually simple. Nonetheless, little emphasis has been placed on preventive treatment or disease-specific therapy, and more could be done even with our present knowledge.
At present, the only drugs available to treat glaucoma are drugs designed to lower IOP. We don't have drugs for neuroprotection, or for treating other aspects of the disease or risk factors such as inflammation and oxidative damage. As a result, most treatment regimens are solely concerned with lowering IOP: If it's 40 mmHg, get it down to 20; if it's 20, get it down to 10. (As the renowned psychologist Abraham Maslow said: If the only tool you have is a hammer, everything looks like a nail!)
However, there are alternative options that may help these patients, currently lumped under the term alternative or complementary medicine, although a preferable term would be non-pharmaceutical medicine. These include Chinese traditional medicine, Ayurvedic medicine and vitamin/nutritional single molecule supplements. These supplements are often potent medications, and their effectiveness in vitro and in animal studies has been shown in numerous systems. These supplements include Ginkgo biloba extract, resveratrol, and curcumin. (Clinical studies can be found by searching the National Library of Medicine/Pubmed website.)
Ginkgo biloba extract, for example, inhibits glutamate activity; blocks NMDA receptors; blocks nitric oxide synthase; and increases blood flow to the brain, the eye and the periphery. (The typical normal tension glaucoma patient is a thin, myopic woman with cold hands and low blood pressure.) Our patients' safety is always of paramount importance, so caution is mandatory. However, many of these supplements have been used by millions of people over the course of centuries, and most have very few side effects. At the least, they deserve our interest and exploration.
Practical Steps in the Clinic
It is still early to talk about how this new perspective regarding glaucoma will eventually change things for the clinician, but the potential ramifications are significant. My current viewpoint is that Alzheimer's disease, macular degeneration and glaucoma exist on a spectrum—they are neurodegenerative disorders with a low-grade inflammatory component, mitochondrial dysfunction, glial activation and oxidative damage. This suggests that what is good for treating one should be good for the others (with the obvious exception of lowering IOP).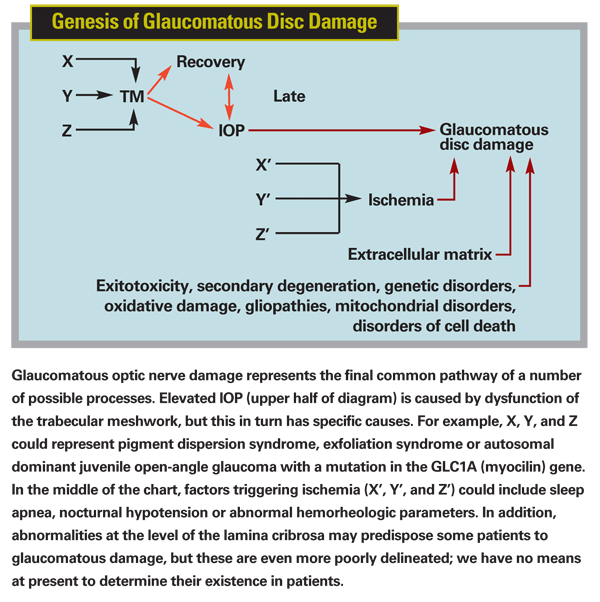
Here are a few things any clinician can consider doing that could prove helpful and won't he harmful:
• Check for and treat high homocysteine levels. High levels are treatable with folate and vitamin B12 supplementation.
• Be observant for systemic issues in NTG patients. People with glaucoma who are progressing at normal IOPs should be investigated for systemic symptoms such as sleep apnea, atrial fibrillation, vasospasm and systemic hypotension.
• Do 24-hour blood pressure monitoring. Like IOP, blood pressure has a diurnal curve, and low pressure at night can be a risk factor for NTG. So, if patients are progressing at what appear to be normal IOP during office hours, it's important to do 24-hour blood pressure monitoring. This is relatively easy because it can be done on an outpatient basis.
• Ask what time of day patients are taking blood pressure medications. Patients who take medications to lower their blood pressure should not be taking them at night. Doing so may cause their pressure to drop lower than intended, a risk factor for normal-tension glaucoma.
• Consider sleep lab testing for apnea. A strong correlation between sleep apnea and glaucoma is increasingly being reported. You can send a patient to a sleep lab for testing and have diurnal blood pressure and IOP measured as well.
• Consider having NTG patients increase their consumption of anti-oxidants. Anti-inflammatory agents may also be valuable, but their relative importance—and which ones might be valuable—remain to be studied. Aerobic exercise is also important, as it lowers the baseline IOP, stimulates endogenous antioxidant production and slows morbidity and mortality in the older population.
A Journey Worth Taking
Alternative approaches to treatment of glaucoma are presently an area of growing activity. One key area of interest is therapy directed toward managing non-pressure-dependent risk factors. Although still in its infancy, this will eventually become an important part of our armamentarium. Risk factor management, for example, is a significant part of cardiologists' approach to treating heart disease, including management of diet, weight, stress, smoking, exercise and cholesterol levels. In the future, we should be able to apply similar principles to the treatment of this disease.
The bulk of this research at present is focused on the status of the blood supply to the optic nerve and posterior pole; the unifying thread is the concept that ischemic changes comprise a significant proportion of such risk factors. (Here again, ischemia can be caused by specific disorders, although they are much less well-known than the anterior segment risk factors.) Another active area of investigation is the concept of secondary degeneration and the use of neuroprotectants to protect the eye against damage from both IOP-dependent- and non-IOP-dependent risk factors.
Clearly, glaucoma is not just a "high pressure disease," nor one necessarily affecting only the eye. Glaucoma in the 21st century is a complex group of diseases with numerous risk factors, ranging from oxidative damage to inflammation to genetic disorders to mitochondrial dysfunction—all of which can lead to apoptosis and glaucomatous disc damage. All of this is just beginning to be elucidated, but the result of this journey will be a vastly improved understand of glaucoma and treatments that will be far more effective and long-lasting.
Dr. Ritch is surgeon director and chief of glaucoma services at the New York Eye and Ear Infirmary, professor of clinical ophthalmology at The New York Medical College,
1. Ritch R, Podos SM. Correspondence re Smith KD, Arthurs BP, Sajeb M: An association between hypothyroidism and primary open-angle glaucoma. Ophthalmology 1993;100:1580-1584, and Ophthalmology 1994;101:623-624.
2. Becker B, Shin DH, Cooper DG, Kass MA. The pigment dispersion syndrome. Am J Ophthalmol 1977;83:2:161-6.
3. Scheie HG, Cameron JD. Pigment dispersion syndrome: A clinical study. Br J Ophthalmol 1981;65:4:264-9.
4. Schlotzer-Schrehardt UM, Koca MR, Naumann GOH, Volkholz H. Pseudoexfoliation syndrome: Ocular manifestation of a systemic disorder? Arch Ophthalmol 1992;110:12:1752-1756.
5. Streeten BW, Li ZY, Wallace RN, Eagle RCJ, Keshgegian AA. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol 1992;110:12:1757-1762.
6. Repo LP, Terasvirta ME, Koivisto KJ. Generalized transluminance of the iris and the frequency of the pseudoexfoliation syndrome in the eyes of transient ischemic attack patients. Ophthalmology 1993;100:3:352-355.
7. Irkec M. Exfoliation and carotid stiffness. Br J Ophthalmol 2006;90:529-530.
8. Aydogan Ozkan B, Yuksel N, Keskin G, et al. Homocysteine levels in plasma and sensorineural hearing loss in patients with pseudoexfoliation syndrome. Eur J Ophthalmol 2006;16:542–547.
9. Shaban RI, Asfour WM. Ocular pseudoexfoliation associated with hearing loss. Saudi Med J 2004;25:1254–1257.
10. Cahill M, Early A, Stack S, Blayney AW, Eustace P. Pseudoexfoliation and sensorineural hearing loss. Eye 2002;16:261–266.
11. Linner E, Popovic V, Gottfries CG, et al. The exfoliation syndrome in cognitive impairment of cerebrovascular or Alzheimer's type. Acta Ophthalmol Scand 2001;79:283–285.
12. Schlotzer-Schrehardt U, Kuchle M, Rummelt C, Naumann GOH. Role of transforming growth factor-b and its latent form binding protein in pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci 1999;40:S278.
13. Thorliefsson G, Magnusson KP, Sulem P, et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science 2007;317:1397–1400.
14. Fingert JH, Alward WL, Kwon YH, Wang K, Streb LM,
15. Hewitt AW, et al. Ancestral LOXL1 variants are associated with pseudoexfoliation in Caucasian Australians but with markedly lower penetrance than in Nordic people. Hum Mol Genet 2008;17:5:710-6.
16. Hayashi H, Gotoh N, Ueda Y, et al. Lysyl oxidase-like 1 polymorphisms and exfoliation syndrome in the Japanese population. Am J Ophthalmol 2008;145:3:582-585.
17. Aragon-Martin JA, Ritch R, Liebmann J, et al. (in press) Evaluation of LOXL1 gene polymorphisms in exfoliation glaucoma. Mol Vis 2008;14:533-41.
18. Oleggini R, Gastaldo N, Di Donato A. Regulation of elastin promoter by lysyl oxidase and growth factors: Cross control of lysyl oxidase on TGF-1 effects. Matrix Biol 2007;26:6:494-505.
19. Pascual G, Mendieta C, Mecham RP, Sommer P, Bellón JM, Buján J. Down-regulation of lysyl oxydase-like in aging and venous insufficiency. Histol Histopathol 2008;23:179-86.
20. Alperin M, Debes K, Abramowitch S, et al. LOXL1 deficiency negatively impacts the biomechanical properties of the mouse vagina and supportive tissues. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:7:977-86.
21. Leibovitch I, Kurtz S, Shemesh G, et al. Hyperhomocystinemia in pseudoexfoliation glaucoma. J Glaucoma 2003;12:36–39.
22. Vessani RM, Liebmann JM, Jofe M, Ritch R. Plasma homocysteine is elevated in patients with exfoliation syndrome. Am J Ophthalmol 2003;136:41–46.
23. Bleich S, Roedl J, Von Ahsen N, Schlotzer-Schrehardt U, Reulbach U, Beck G, Kruse FE, Naumann GOH, Kornhuber J, Junemann AGM. Elevated homocysteine levels in aqueous humor of patients with pseudoexfoliation glaucoma. Am J Ophthalmol 2004;138:162–164.
24. Puustjarvi T, Blomster H, Kontkanen M, et al. Plasma and aqueous humour levels of homocysteine in exfoliation syndrome. Graefe's Arch Clin Exp Ophthalmol 2004;242:749–754.
25. Altintas O, Maral H, Yuksel N, Karabas VL,
26. Roedl JB, Bleich S, Reulbach U, et al. Homocysteine in tear fluid of patients with pseudoexfoliation glaucoma. J Glaucoma 2007;16:234–239.
27. Roedl JB, Bleich S, Reulbach U, et al. Vitamin deficiency and hyperhomocysteinemia in pseudoexfoliation glaucoma. J Neural Transm 2007;114:571–575.
28. Raposo B, Rodriguez C, Martínez-Gonzáles J, Badimon L. High levels of homocysteine inhibit lysyl oxidase (LOX) and downregulate LOX expression in vascular endothelial cells. Atherosclerosis 2004;177:1-8.
29. Abu El-Asrar AM, Abdel Gader AG, Al-Amro SA, Al-Attas OS. Hyperhomocysteinemia and retinal vascular occlusive disease. Eur J Ophthalmol 2002;12:495-500.
30. Hankey GJ, Eikelboom JW. Homocysteine and stroke. Curr Opin Neurol 2001;14:95-102.
31. Morris MS. Homocysteine and Alzheimer"s disease. Lancet Neurol 2003;2:425-428.
32. Clarke R. Commentary: An updated review of the published studies of homocysteine and cardiovascular disease. Int J Epidemiol 2002;31:70-71.