Medical procedures move from experimental to investigative to commonplace when they are shown to deliver better outcomes at comparable costs than existing approaches. A keratoprosthesis has been recognized for its potential to restore vision in patients with corneal blindness who would typically be poor candidates for routine corneal allografting, and our group at Massachusetts Eye and Ear Infirmary recently documented that this device can be as cost-effective as a penetrating keratoplasty in treating severely diseased corneas.1
Claes H. Dohlman, MD, PhD, is the developer of the Boston Keratoprosthesis, or Boston Kpro. This PMMA and titanium-composed artificial cornea has been available commercially since 1992. There is no comparable device at the moment. A host of studies over the past few years have validated the
Old Idea, New Prosthetic
The concept of a prosthetic cornea actually goes back hundreds of years. However, it wasn't until the development of PMMA and the revelation that the eye tolerated its properties well that investigators turned their attention toward using this material to replace a damaged cornea. In 1949, Sir Harold Ridley implanted the first intraocular lens made of PMMA in a cataract patient in
Encouraged by the results of PMMA IOLs, Dr. Dohlman began work on a PMMA-based prosthetic cornea about 40 years ago. After he retired from his position as chair of ophthalmology at
At first, Dr. Dohlman limited use of the device to a few patients. He encountered a fair number of failures, but changes to the device and perioperative care continued to improve clinical outcomes. Use of the Boston Kpro really began to grow substantially only in the last decade, and took off nationally and internationally in the past two to three years. Last year more than 1,200 Boston Kpro devices were implanted.
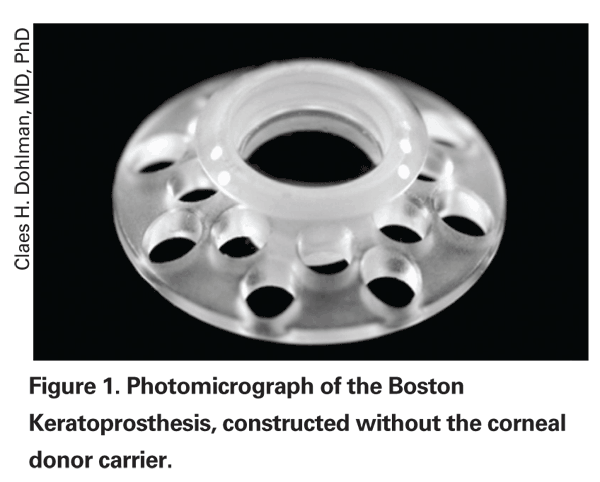
Today the Boston Kpro is made of two parts PMMA and one part titanium (See Figure 1). The single-piece stem and front plate are placed through a 3-mm trephine incision in a donor or the patient's own cornea to join with the PMMA backplate. Finally, a titanium locking ring is snapped into place on the back of the stem behind the backplate, securing the device to the cornea. The cornea-Kpro construct is then sutured into the corneal recipient bed as in standard penetrating keratoplasty, and a contact lens is placed to prevent surface drying and dellen formation. There is a pending 510(k) FDA application to replace the backplate, currently lathed from PMMA, with one made from titanium. We are seeking this change because of recent reports suggesting that titanium will induce less retroprosthetic membrane formation.
Indications and Uses
The Boston Kpro has been a godsend to patients with repeated corneal allograft rejection after PK. While corneal transplantation has had excellent results overall, graft rejection can be a nightmare for both the patient and surgeon. With each attempted retransplantation, the rejection risk increases and the overall success rate declines—to the point where a third corneal allograft has about a zero percent survival rate at five years. Therefore, keratoprosthesis surgery has proved to be a good option to treat patients after multiple repeat corneal allografts.
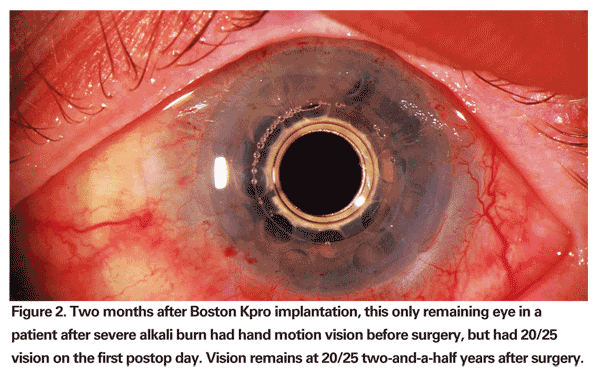
Because of the optical clarity of the Boston Kpro, we have also found it can afford very early recovery of vision (See Figure 2). Some patients recover normal vision fully on the first day after surgery. After corneal transplant, visual recovery can take months or even years, and vision can be compromised by dryness and astigmatism. The spherical curvature of the keratoprosthesis obviates issues of astigmatism. Ocular surface dryness has only been a concern in patients with severe ocular surface disease.
The Boston Type 1 Keratoprosthesis Study Group investigated several additional applications of the device: chemical injury, bullous keratopathy and herpes simplex virus keratitis.3 The study involved 136 eyes. Of a subgroup of 62 eyes followed for at least one year after keratoprosthesis implantation, 56 percent had BCVA of 20/200 or better and 23 percent had BCVA of 20/40 or better. That compared favorably with a preoperative BCVA of 20/200 or better among 4 percent of the entire study group.
A group at University of California-Davis reported on 30 eyes that underwent keratoprosthesis implantation in 28 patients.4 Before the operations, only 17 percent of patients had BCVA of 20/200 or better compared with 77 percent postoperatively. Among the 16 eyes followed for at least a year, 12 eyes, or 75 percent, had BCVA of 20/200 or better. The list of postoperative complications among this group was similar to that of the Boston Type 1 Keratoprosthesis Study Group.
A single-surgeon study of 50 eyes in 49 patients out of Jules Stein Eye Institute at UCLA found similar results and complications.5 Follow-up went out to three years in this study, and the authors found that the percentage of eyes with BCVA of 20/100 or better actually increased, from 75 percent at 12 months to 100 percent at 36 months. In this study, as with others, a large percentage of eyes had two or more previous corneal transplantations.
Evolving Indications
Indications for the keratoprosthesis continue to evolve. Patients with aniridia have poor results after corneal transplantation because of the aniridic cornea's high vascularity and difficulty with epithelial healing. The Boston Kpro has been used a first-line treatment in patients with aniridic keratopathy with good results.
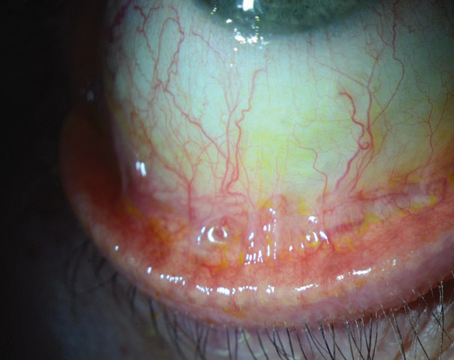
The Boston Kpro has also been used in patients with chemical corneal injuries, with mixed results. This may be due to uncontrolled tissue inflammation and an increased tendency for corneal stromal melts in these patients. It should be noted that patients with mucous membrane pemphigoid and Stevens-Johnson syndrome, and those after severe alkali injury have the worst outcomes with the Boston Kpro. However, these are also patients in whom a standard corneal allograft would fare even more poorly.
Issues and Complications
Use of the Boston Kpro is not without its complications. In the Boston Type 1 Keratoprosthesis Study Group, 35 of the 136 eyes developed retroprosthetic membranes; of which 26 were treatable with YAG laser membranotomy and four required surgical membranectomy (the others required no treatment).3 Other nonsurgical complications this group encountered with the keratoprosthesis included pressure spikes (21), vitritis (7), vitreous hemorrhage (6) and retinal detachment (5).3
The PMMA keratoprosthesis creates a rigid surface in the front of the eye. This means that contact tonometry is of no use in evaluating the intraocular pressures of these patients. This limits the surgeon to tactile estimation of intraocular pressure, best performed through the upper eyelid with the patient looking down. Glaucoma is common in patients with corneal disorders that bring them to the keratoprosthesis, and following their IOP after implantation of the device is more difficult.
Patients with severe alkali injuries or autoimmune disorders are at increased risk for retinal detachment after implantation of the keratoprosthesis, possibly due to their propensity to develop vitreous inflammation after surgery. These patients have the worst prognosis with the Boston Kpro, but they have near-zero prognosis with PK. Whether such patients should receive the Boston Kpro is a frequent discussion among corneal specialists.
Besides the keratoprosthesis, another option for alkali injured patients or those with autoimmune diseases would be limbal stem cell allograft surgery. However, no consensus exists regarding who should receive one or the other. We are trying to understand how to optimize Kpro outcomes in these high-risk patients, through a systems approach that encompasses reevaluation of the device itself, the surgical procedure—particularly whether such patients should have subtotal vitrectomy and glaucoma valve placement concurrent with Kpro implantation—and the perioperative use of systemic immune suppressive and topical antifungal medications.
We expect the Boston Kpro to become more widely used in the future. Our paper on the cost-effectiveness of the device was significant for two reasons: Corneal transplantation is done in essentially healthy eyes with corneal disorders, whereas the keratoprosthesis is used in more challenging eyes that have either failed at transplant or are not candidates for it; and the cost-effectiveness of both procedures is comparable.
Dr. Chodosh is lecturer in the Department of Ophthalmology,
1. Ament JD, Stryjewski TP, Ciolino JB, Todani A, Chodosh J, Dohlman CH. Cost-effectiveness of the Boston keratoprosthesis. Am J Ophthalmol 2010;149:221-228.
2. Obituaries: Sir Harold Ridley. The Independent. June 13, 2001. Available at: http://www.independent.co.uk/news/obituaries/sir-harold-ridley-729214.html
3. Zerbe BL, Belin MW, Ciolino JB for the
4. Bradley JC, Hernandez EG, Schwab IR, Mannis MJ.
5. Aldave AJ, Khairidzan MK, Vo RC, Yu F. The
s.