Having the right drug to treat an internal ocular problem is only half the battle. The other half is getting the drug safely and effectively into the eye. Two new technologies under development may do just that—far more effectively than drops, and with fewer risks and limitations than injections or inserts.
Driving Drugs Through the Sclera
The EyeGate II iontophoresis drug delivery device uses electrochemical repulsion to drive charged drug molecules out of a solvent solution and into the eye. The instrument features a small ocular applicator that's hollow in the center, with a doughnut-shaped foam reservoir that holds the solution containing the drug. The foam reservoir "doughnut," which is about 1 mm wide where it touches the eye, rests against a circular swatch of sclera and conjunctiva just outside the limbal region. The applicator is connected to a programmable generator, which is in turn connected to an electrode that's placed on the forehead of the patient. During treatment, the generator creates an electrical field inside the applicator and an opposite electrical charge on the patient's forehead; the resulting electrochemical repulsion within the applicator drives like-charged drug molecules out of the solution and into the eye. The programmable generator allows the doctor to control the speed of delivery and amount of drug delivered.
Mike Patane, PhD, chief scientific officer at Eyegate Pharma (Waltham, Mass.), explains that each formulation used in the device is customized for the drug it's delivering. "Every drug molecule has different physicochemical characteristics and requires a slightly different formulation," he says. "Most molecules have an inherent charge, so we establish the pH range at which that molecule is most likely to be in an ionized state; then we tailor the solution pH appropriately. The more drug molecules are ionized, the more drug delivery is achieved." He notes that being ionized doesn't affect the functionality of the drug.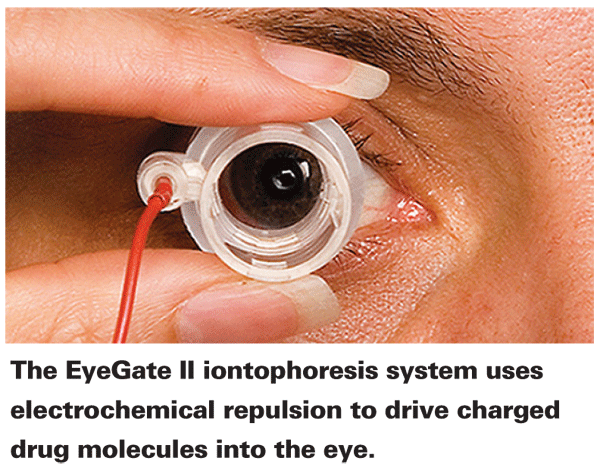
Dr. Patane explains that the foam reservoir in the applicator is designed to rest against the sclera and conjunctiva for several reasons. "The sclera has much larger pores and greater pore density than the cornea, which is more like a barrier," he notes. "The sclera also has fewer nerves than the cornea, so the patient feels less sensation. This location also allows us to deliver drugs to both the anterior chamber and vitreous cavity." He adds that the literature indicates that pore size and density within the sclera are highly consistent across different patient types and age groups; as a result, they haven't found significant variations in delivery between patients. Also, the sclera has very limited capacity to degrade molecules that pass through it, so drugs make it into the eye without significant alteration.
Practical Advantages
"Our highest goal is to replace the need for ocular injections," says Dr. Patane. "In animal studies we've been able to deliver as much or more drug through the ocular surface as can be achieved with an ocular injection. And while injections can produce an IOP spike, we haven't seen any spikes following our procedure. One reason for this is that an injection pushes the carrier solution into the eye along with the drug; our device only drives the drug molecules in, leaving the solution in the applicator. This causes almost no volume increase inside the eye."
Dr. Patane says the company believes this approach to drug delivery could be less expensive than injections. "Also, for some acute diseases, our data suggests that we may be able to deliver enough drug in a single treatment to make longer-term daily regimens unnecessary. That means potentially fewer visits to the doctor for follow-up and less need for longterm prescription drug use—plus, more quick impact for the patient."
The initial clinical trials of the delivery system are also evaluating the efficacy of a new proprietary drug, EGP- 437. "EGP is a corticosteroid solution that was custom formulated for delivery with our system," he explains. "We've been able to demonstrate that we can deliver substantial amounts of the drug to the anterior and posterior chambers. The drug is being studied in both dry-eye patients, where we've completed a Phase II clinical trial, and in patients with anterior uveitis." (The intention in the case of dry eye is to drive the drug into the ocular surface, which then acts as a depot.)
"In the dry-eye trial we've been able to show rapid onset of action, improvement in signs and symptoms, and effects that lasted through the three-week study period," he continues. "In the anterior uveitis study, the data showed rapid decreases in anterior cell chamber count that wouldn't have been achievable with topical drop approaches. Four of six of Dr. Victor Perez's patients at the Bascom Palmer Eye Institute achieved an anterior cell score of zero by seven days; by day 14, all six had a score of zero."
Dr. Patane says the company anticipates completing clinical trials within 12 to 18 months; they hope to achieve U. S. Food and Drug Administration approval a year or two after that. In terms of cost, he says the initial investment for the physician should be minimal. "The generator for the device costs less than $1,000z," he notes. "We hope that the drug product and disposable elements such as the applicator and return electrode will all be covered together under one j-code, with the reimbursement dependent on the cost of the drug being delivered."
A Programmable Pump
Retinal surgeon Mark S. Humayun, MD, PhD, is a professor of ophthalmology and biomedical engineering at the Doheny Eye Institute at the University of Southern California in Los Angeles, and director of both the National Science Foundation's National Center of Excellence in Engineering and the U.S. Department of Energy's artificial retina project. Currently, working with a spinoff company from the engineering center, his team has developed a sophisticated electronic pump for ocular drug delivery. The device, which is controlled by a tiny onboard computer chip, is designed to be attached to the sclera under the conjunctiva, placed posteriorly, about where the muscles attach to the eye. The device has a delivery cannula that enters the eye, and it includes a refillable reservoir to hold a supply of the drug.
"This device is totally programmable, so it can respond to variations in eye conditions," notes Dr. Humayun. "It can contain enough drug to allow delivery for months, and you can refill it, which can allow it to function for years. You can switch drugs without switching the device, but it's easy to implant and remove." He adds that the size of the device depends on the volume of drug it contains. "Most variations holding enough drug to last for several months would probably be the size of an Ahmed drainage device used to treat glaucoma," he says.
"To refill the reservoir, you connect to the device using a proprietary automated refill system with a very small 30-ga. Conduit similar to a syringe—although there's nothing to push or pull," he explains. "You align the conduit until you hear a click and see a green light. The device flushes the reservoir to clean out any residual drug and then refills it within 10 to 30 seconds. During the same interface, it repowers the pump, while allowing you to change the delivery programming as necessary. You can also download time-stamped data showing how much drug was delivered and when. The charging and programming may take 10 or 20 minutes, but all of this can be done in the clinic."
To be sure the pump is refilled in a timely manner, Dr. Humayun says the most basic approach is simply to schedule a return appointment before the reservoir will be empty. "If the patient doesn't come in, auditory or visual indicators warn the patient that the device is running out of the drug," he explains. "If the patient ig-nores all of that, at some point the pump will automatically shut down. And, if there's a malfunction, it will automatically shut down and beep intermittently until you tend to it."
Small But Powerful
Dr. Humayun explains that the pump uses electrolysis to move the fluid. "Splitting water into hydrogen and oxygen results in a thousandfold expansion of volume," he says. "As a result, this tiny pump is capable of generating 600 mmHg or more of pressure with only microwatt power consumption. This is important for several reasons: First, it allows the pump to be very small; second, even high pressure inside the eye won't prevent the drug from being delivered; and third, no clog in the tube will be able to block the flow. Trying to accomplish this with a peristaltic or piston pump would consume 100 times more power and require a pump the size of a hockey puck."
Dr. Humayun points out that the quantity of drug being pumped into the eye can be miniscule. "We're only delivering nanoliters of drug, which is far too little to cause any increase in IOP," he says. "In animal trials we've already seen that a beta blocker delivered using the pump has been able to lower IOP just as much as 100 times the quantity of the same beta blocker administered in an eyedrop."
Are there limitations to the drugs that could be delivered using this technology? Dr. Humayun says the main concerns are about what might happen when a drug sits in the reservoir for months. "Large molecules might precipitate out of solution over time," he notes. "So some suspensions would need to be tested for this. However, Avastin is a huge molecule, and it didn't precipitate out during four months inside the reservoir. In addition, the eye moves a lot during the day, so we have a built-in stirring mechanism."
Dr. Humayun says that how soon the pump might be commercially available depends mostly on the regulatory process. "The device will be ready for clinical trials outside the United States next year," he notes.



