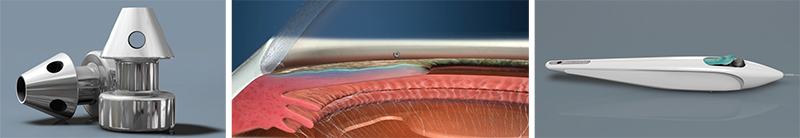
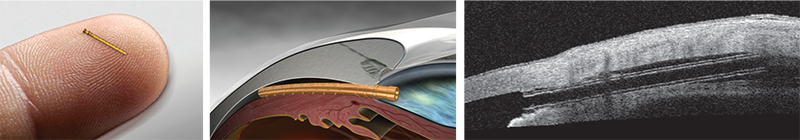
The iStent Inject
Most ophthalmic surgeons are familiar with the Glaukos iStent. One of two new iterations of this device, the iStent Inject, is now in clinical trials outside the United States. (The other, the iStent Supra, targets uveoscleral outflow and is also under investigation.) L. Jay Katz, MD, director of the Glaucoma Service at Wills Eye Hospital in Philadelphia, has experience using the iStent Inject.
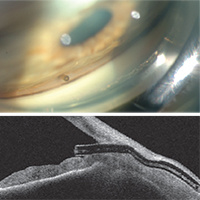
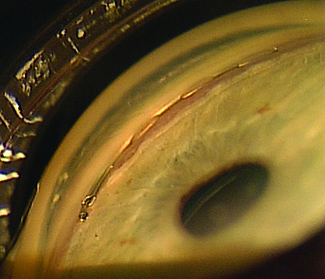
Dr. Katz points out several differences between the currently available iStent and the iStent Inject. “Instead of being an L-shape, the second-generation device is shaped a little bit like a mushroom,” he says. “Placement technique is also different. Instead of a venipuncture-like technique, the iStent Inject uses a straight-on approach, a little bit like a nail gun. You go directly onto the meshwork with the forceps delivery and push the little mushroom stent directly through the meshwork and into Schlemm’s canal. Technically it’s easier because you’re going directly into where you think Schlemm’s canal is, head-on, instead of trying to cannulate it.
“Another difference is that this device can be loaded with multiple stents,” he continues. “Currently, two stents are preloaded into one delivery device. So you push one stent in, then move over and push the second stent in without exiting the eye. The nice thing about multiple stent placement is that we now have a multitude of studies showing that multiple stents do produce a lower IOP than a single stent does. That makes sense, since the outflow apparatus of the eye is circular and you have collector channels all along the perimeter draining from Schlemm’s canal. The more access to the collector channels you have with multiple stents, the more pressure-lowering you’d expect, and the studies thus far indicate that this is the case.”
|
Dr. Katz explains that although the device is experimental in the United States, he has implanted the iStent Inject as part of a multi-surgeon trial in Armenia. “As part of the protocol we were implanting both iStent versions in different patients, so it was easy to compare them,” he says. “There’s no question that, in my hands at least, the iStent Inject is a lot easier to use.”
A recent randomized study sponsored by Glaukos compared treating open-angle glaucoma with a fixed combination of latanoprost/timolol vs. implantation of two iStent Inject devices in 192 subjects.1 Both groups displayed a high safety profile, and both produced significant reductions (≥20 mmHg) in IOP. However, there was a 17.5-percent, statistically significant between-group difference in favor of the iStent Inject at the ≥50 percent level of IOP reduction. The authors concluded that using the iStent Inject was at least as effective as using two medications.
“Any time you have a new surgery, there’s always a learning curve,” adds Dr. Katz. “However, if you’ve already used the first version of the iStent you’ll be ahead of the curve. A lot of the surgical novelty when using an iStent has to do with positioning the patient, holding a gonio lens, using different hand positions and inserting the device. All of these are essentially the same with the second-generation stent. The only thing that’s a little different is that you have to learn how to push it into place just the right amount, and that requires some learning. But it’s certainly a faster learning curve than with the first device.”
The Xen Gel Stent
| ||||||
“Most MIGS devices are designed to enhance normal aqueous outflow through the trabecular meshwork and Schlemm’s canal,” he notes. “This stent is different in that it bypasses the natural drainage pathway and can produce the lower intraocular pressures that we typically only get with trabeculectomy or tube shunt procedures. However, there are numerous potential advantages of the Xen gel stent over a trabeculectomy or tube shunt. It requires only 15 to 20 minutes to implant, gives you immediate intraocular pressure reduction and needs relatively little postoperative management. The small luminal diameter of this shunt seems to provide enough resistance to aqueous flow that postoperative hypotony is minimized, and this procedure may be combined with a subconjunctival injection of mitomycin-C to enhance long-term intraocular pressure control. Of course, like any new procedure, there is a learning curve that must be overcome at the outset.”
Dr. Panarelli says that despite some similarity to trabeculectomy, the Xen gel stent can qualify as a MIGS procedure. “I believe it meets the five criteria specified by Ike Ahmed,” he says. “It’s inserted through a clear corneal microincision; it’s minimally traumatic to the targeted tissue; it allows for rapid recovery with minimal impact on the patient’s quality of life; and it has a good safety profile. The fifth criteria, efficacy, will be determined by the ongoing trials of the device, but having implanted it myself, I’ve seen that we can get reasonable intraocular pressure reduction with it. This device has the potential to help bridge the gap between medical treatment and filtering/tube shunt surgery.”
Inserting the Xen Gel Stent
Dr. Panarelli says that to perform the basic procedure he begins sitting temporally. “I make a 2-mm temporal clear cornea incision about 180 degrees from where I want the device to be seated in the superonasal quadrant,” he explains. “Then I take the inserter, which is similar to an IOL inserter, and enter the anterior chamber. The 27-ga. needle inserter tip should pierce the anterior portion of the trabecular meshwork; it is then advanced forward until it exits the sclera. The ideal ‘landing spot’ is 3 mm posterior to the limbus. While the implant is being delivered into the subconjunctival space, the needle slowly retracts. Gentle forward pressure is applied to keep the device from springing back into the anterior chamber. Once the stent is in place, a bleb will begin to form immediately.
“Good preoperative gonioscopy is essential to successful implantation,” he notes. “You need to make sure you have at least a grade-three open angle where you want to implant the device; you don’t want a narrow angle or focal peripheral anterior synechiae. I always perform the procedure under a retrobulbar block because the patients may feel some pain when you insert and advance the device through the sclera.”
Dr. Panarelli says the learning curve is similar to the other MIGS procedures, but may be a little easier. “Many of those procedures have to be done under direct gonioscopy; this one doesn’t,” he notes. “I do perform indirect gonioscopy to confirm where the needle is seated in the angle before advancing the device, but it’s not a required part of the procedure. With experience, this procedure can be performed quickly and safely, but I suspect it will more often be performed by glaucoma surgeons rather than cataract surgeons as it may be better-suited to treating patients with moderate-to-advanced glaucoma.”
The Hydrus Microstent
| ||||
Dr. Saheb says there are no published studies on the clinical efficacy of the Hydrus device yet. “However, there have been some basic science studies [sponsored by Ivantis],” he says. “The cadaver Schlemm’s canal integrity study, which was a scanning electron microscopy study of Schlemm’s canal in post-mortem eyes after insertion of the Hydrus device, showed integrity of the collector channels in the area of the Hydrus insertion.2 Another study showed increased outflow in post-mortem human eyes after insertion of the Hydrus device at multiple IOP levels.3 There was also a biocompatibility study which looked at inflammation and encapsulation after insertion of the Hydrus device in both nonhuman primates and rabbits; months after insertion the devices had caused minimal inflammation or encapsulation.4 These three published studies suggest a good safety profile for this device, and promising effect.”
Dr. Saheb adds that a number of clinical studies are currently under way, although none have been published in the peer-reviewed literature. “However, data reported at the meetings from randomized clinical trials and case series indicates that all the studies have so far found a significant reduction in IOP and medications after combined cataract surgery and Hydrus device implantation,” he says. “They have also reported a safety profile similar to that of cataract surgery alone. Hopefully, the first randomized, controlled trial results will be published in the next year or two.”
Dr. Saheb says he has implanted a number of the Hydrus stents himself. “My experience has been very positive,” he says. “As with most ab interno surgeries, there is a learning curve to inserting the device properly, but that can be quite quick for those who have prior gonioscopic surgical skills.”
The CyPass Micro-Stent
Dr. Saheb has also had limited experience with the CyPass Micro-Stent (Transcend Medical). “This is another ab interno glaucoma device,” he says. “It’s inserted through the angle into the supraciliary space, designed to take advantage of the negative pressure gradient between the suprachoroidal space and the anterior chamber. It’s made from a polyimide material; it’s 6.35 mm long, with an external diameter of 510 µm.”
Dr. Saheb says the insertion is relatively simple compared to other ab interno devices. “You aim to place the device between the scleral spur and the ciliary body,” he says. “It then slides along this potential space between the scleral spur, which becomes the internal scleral wall posteriorly, and the ciliary body, which becomes the choroid posteriorly. The potential space between the choroid and the sclera follows along that plane, and the junction of the scleral spur and the ciliary body is readily visible in the gonioscopic view. I found the surgery to be very straightforward.
|
“In terms of data, a recently published clinical study looked at 142 patients with open-angle glaucoma and cataract who underwent device implantation,” he adds.5 “They separated the cohorts into patients with a high baseline IOP and those with a low baseline IOP. They found that in the group with high baseline IOP there was a significant lowering of the IOP and number of medications. In the group with low baseline IOP, there was stabilization of IOP and a lowering of the number of meds. The safety profile was comparable to the safety of cataract surgery alone.” REVIEW
1. Fea AM, Belda JI, Rkas M, Jünemann A, Chang L, Pablo L, Voskanyan L, Katz LJ. Prospective unmasked randomized evaluation of the iStent inject versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol 2014;8:875-82. doi: 10.2147/OPTH.S59932.
2. Johnstone MA, Saheb H, et al. Effects of a Schlemm’s canal scaffold on collector channel ostia in human anterior segments. Exp Eye Res. 2014;119:70-6.
3. Camras LJ, Yuan F, et al. A novel Schlemm’s Canal scaffold increases outflow facility in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci. 2012 Sep 12;53:10:6115-21. doi: 10.1167/iovs.12-9570.
4. Grierson I, Saheb H, Kahook MY, et al. . A Novel Schlemm’s Canal Scaffold: Histologic Observations. J Glaucoma. 2013 Nov 14. [Epub ahead of print]
5. Hoeh H, Vold SD, et al. Initial Clinical Experience With the CyPass Micro-Stent: Safety and Surgical Outcomes of a Novel Supraciliary Microstent. J Glaucoma. 2014 Oct 9. [Epub ahead of print].