Workup, Diagnosis and Treatment
The most common causes of spontaneous vitreous hemorrhage in adults include proliferative diabetic retinopathy, retinal tear, posterior vitreous detachment, retinal detachment, veno-occlusive disease and exudative age-related macular degeneration.1,2 Less-common causes include retinal macroaneurysm, proliferative sickle cell retinopathy, hematologic disorders, vascular diseases (hypertensive retinopathy, ocular ischemic syndrome) and infectious/inflammatory etiologies (pars planitis, ocular toxocariasis, lupus vasculitis).1 In cases of unilateral dense vitreous hemorrhage, dilated fundoscopic examination of the fellow eye can provide clues to diagnosis. In this patient with known diabetes mellitus, examination of the fellow eye revealed no signs of diabetic retinopathy, macular degeneration or other vascular abnormalities.
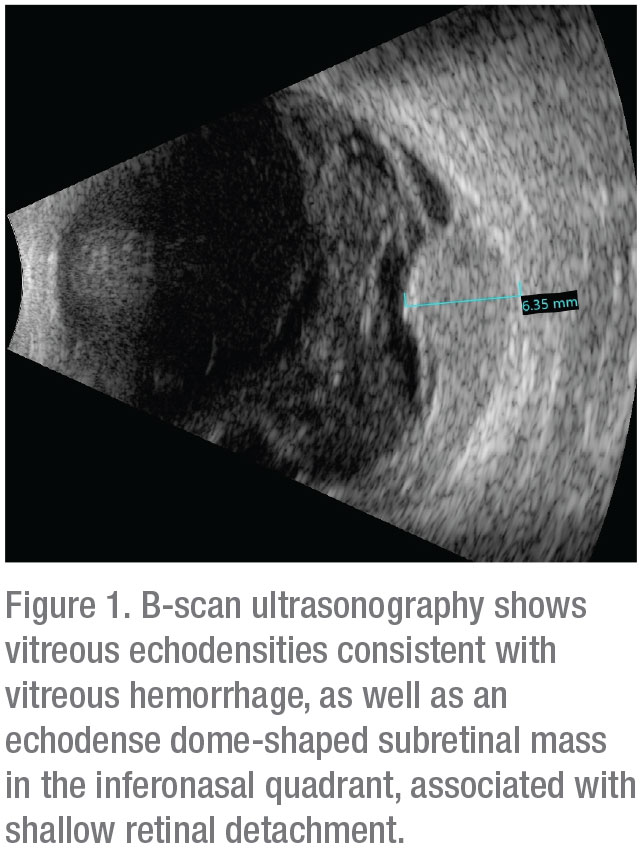 |
B-scan ultrasonography confirmed presence of vitreous hemorrhage with posterior vitreous detachment and also revealed an echodense dome-shaped subretinal mass inferonasally measuring 6.4 mm in thickness. A surrounding shallow retinal detachment and questionable spontaneous vascular pulsations indicated that it was most likely a solid tumor with intrinsic vessels, and not simply a hemorrhage (Figure 1). No extraocular extension was noted. Globe transillumination revealed a dense shadow in the anterior choroid between the 4 and 5 o’clock meridians that partially extended into the pars plana of the ciliary body. These findings on transillumination were further evidence that this was a solid tumor, as vitreous or subretinal hemorrhage would typically stop at or near the ora serrata, whereas a solid tumor can extend beyond that into the ciliary body. Ultrasound biomicroscopy confirmed an echodense mass in the pars plana region with no anterior chamber involvement (Figure 2). Given the results of ultrasonography and transillumination, the possibility of a ciliochoroidal tumor was highly suspected.
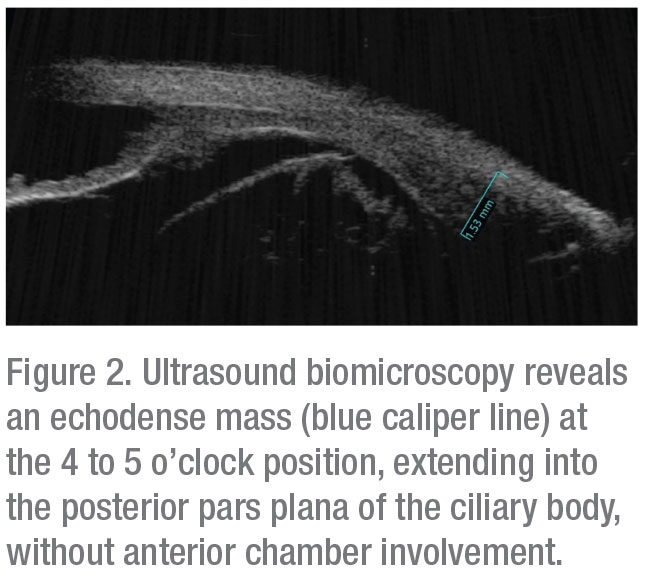 |
Choroidal melanoma, retinoblastoma, retinal cavernous hemangioma, retinal astrocytic hamartoma and vasoproliferative tumors of the retina can, in some cases, present with intraocular bleeding. On the other hand, choroidal hemangioma, choroidal osteoma, choroidal or vitreoretinal lymphoma and choroidal metastasis are not likely to present with hemorrhage.
Magnetic resonance imaging of the orbits was subsequently performed; it showed a solitary, well-defined enhancing lesion in the inferior nasal quadrant of the right eye. The lesion was hyperintense on T1-weighted images and hypointense on T2-weighted images; it showed moderate enhancement following contrast administration (Figure 3).
The findings and suspicion of uveal melanoma were discussed with the patient; he preferred conservative observation to see if clearing of the vitreous blood might allow improved visualization. At the follow-up visit at four weeks, visual acuity had improved to 20/30 in the affected eye and vitreous hemorrhage was resolving, which permitted observation of a pigmented subretinal mass in the inferonasal fundus, with associated serous retinal detachment (Figure 4). On B-scan ultrasonography the tumor thickness had decreased to 5.1 mm. The decrease in tumor thickness on ultrasonography since initial presentation raised the possibility of subretinal or choroidal hemorrhage (Figure 5), although a hemorrhagic choroidal melanoma remained at the top of the differential diagnosis. To confirm the diagnosis, fine needle aspiration biopsy of the subretinal mass was performed. Cytopathology demonstrated a few pigmented spindle B melanoma cells with prominent nucleoli, as well as amorphous necrotic debris, consistent with necrotic malignant melanoma. On follow-up examination five weeks after the original presentation, the tumor had decreased further in size and was measured to be 4.3 mm in thickness by ultrasonography. The patient underwent I-125 plaque brachytherapy of the choroidal tumor in conjunction with fine needle biopsy of the tumor for cytogenetic evaluation. He is scheduled to receive six intravitreal injections of bevacizumab at four-month intervals over the next two years, and to undergo prophylactic sector scatter laser photocoagulation to the retina surrounding the tumor to reduce the potential for vision loss after radiation.
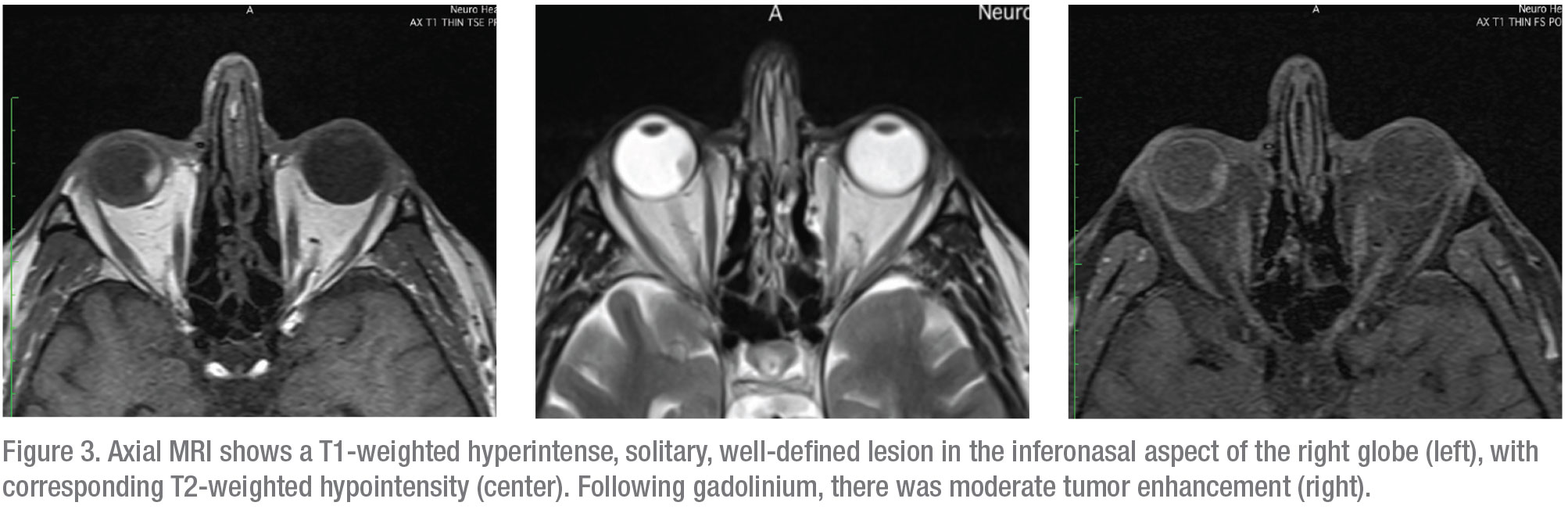 |
Discussion
Choroidal melanoma is the most common primary intraocular malignancy in the United States. Choroidal melanomas can occasionally present with vitreous hemorrhage. In a study on 8,033 cases of uveal melanoma, the overall incidence of intraocular hemorrhage at the time of presentation was 10 percent, but this finding increased with increasing patient age (8 percent in patients <20 years, 9 percent in patients 21 to 59 years, and 12 percent in patients >60 years).3
The incidence of spontaneous necrosis of choroidal melanoma is thought to be between 3 and 9 percent.4,5 As compared to non-necrotic tumors, necrotic choroidal melanomas are more likely to present with inflammatory exophthalmos, secondary angle-closure glaucoma, iris neovascularization, episcleritis, scleritis and uveitis.5,6 Other presenting signs and symptoms include pain, proptosis, orbital inflammation, ophthalmoplegia, conjunctivitis, hyphema, uveal effusion, vitreous hemorrhage and increased intraocular pressure.7,8,9,10,11
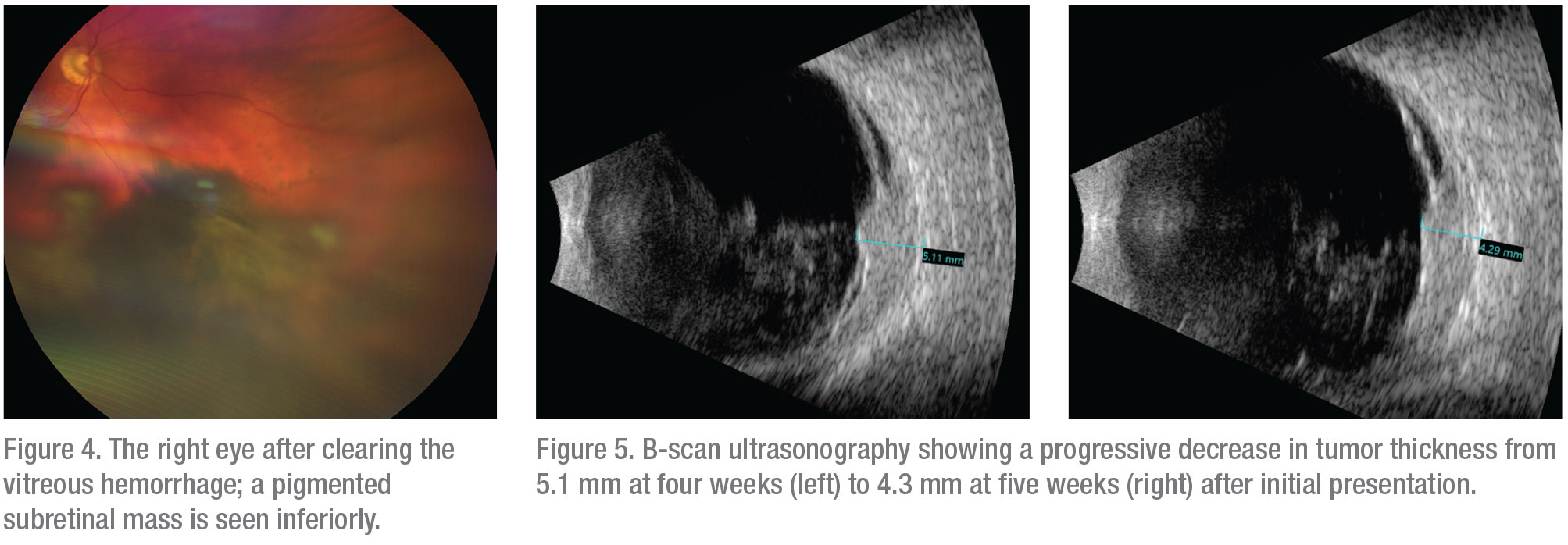 |
Several mechanisms have been proposed to explain the pathogenesis of spontaneous necrosis of a choroidal melanoma. Some postulate that the mass effect of the tumor leads to anterior displacement of the iris, causing acute angle closure and increased intraocular pressure. That, in turn, results in vascular compromise and ischemic necrosis of the tumor.8 Others propose that the enlarging tumor outgrows its blood supply, leading to hypoxia and ischemic necrosis in the tumor’s center. Secondary inflammation and tumor swelling can eventually result in anterior lens displacement and secondary angle-closure glaucoma, further potentiating tumor necrosis and a generalized ischemia and necrosis of the iris and ciliary body.11 Another proposed mechanism places the emphasis on tumor vasculitis as the initiating factor.8 These mechanisms explain the increased incidence of pain, inflammatory signs and increased IOP in eyes with necrotic choroidal melanomas.
As evidenced by the present case, necrotic choroidal melanomas can present a diagnostic challenge. These tumors can appear acoustically dense, as compared to tumors without necrosis, which are usually somewhat hollow on ultrasonography. These dense echoes may be explained by tumor necrosis and intratumor hemorrhage. Unlike non-necrotic tumors that are classically contrast-enhancing on MRI, necrotic tumors can show minimal to no enhancement secondary to necrosis.12,13 Additionally, the decreasing size of the neoplasm on ultrasonography, as the tumor undergoes necrosis, may be mistaken for resolving hemorrhage. Care should be taken to differentiate the two diagnoses.
Additional challenges are encountered when this tumor presents with vitreous hemorrhage. Dense hemorrhage not only makes direct visualization of the tumor difficult, but can also preclude identification of the tumor shadow on globe transillumination. In these cases, the distinct tumor shadow may be absent and the choroid may appear diffusely dark.12 Additionally, subretinal or suprachoroidal hemorrhage with ensuing echoes can obscure the tumor on ultrasonography.12 Although the presence of spontaneous vascular pulsations on ultrasonography is an important clue to the diagnosis of choroidal melanoma vs. hemorrhage, this finding may be absent in cases of extensive necrosis.12,14 Subacute hemorrhage can also obscure choroidal melanoma identification on MRI, as both appear hyperintense on T1-weighted sequences.12 A T2-weighted MRI image may be helpful in differentiating a tumor from an evolving hemorrhage.12,13
In conclusion, although it’s a rare cause of intraocular hemorrhage, choroidal melanoma should be considered in the differential diagnosis in patients presenting with spontaneous vitreous hemorrhage and a subretinal mass. Special care should be taken to differentiate intraocular hemorrhage from a necrotic choroidal melanoma, as the two share several common features on ancillary testing. REVIEW
1. Spraul CW, Grossniklaus HE. Vitreous hemorrhage. Survey of Ophthalmology 1997;1:42:1:3-9.
2. Conart JB, Berrod JP. Non-traumatic vitreous hemorrhage. Journal Francais D’Ophtalmologie. 2016;39:2:219-25.
3. Shields CL, Kaliki S, Furuta M, Mashayekhi A, Shields JA. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina 2012;32:7:1363-72.
4. Paul EV, Parnell BL, Fraker M. Prognosis of malignant melanomas of the choroid and ciliary body. Int Ophthalmol Clin 1962;2:387–402.
5. Bujara K: Necrotic malignant melanomas of the choroid and ciliary body. A clinicopathological and statistical study. Graefes Arch Clin Exp Ophthalmol 1982;219:40–43.
6. Moshari A, Cheeseman EW, McLean IW. Totally necrotic choroidal and ciliary body melanomas: Associations with prognosis, episcleritis, and scleritis. Am J Ophthalmol 2001;131:232–236.
7. Palamar M, Thangappan A, Shields CL, Ehya H, Shields JA: Necrotic choroidal melanoma with scleritis and choroidal effusion. Cornea 2009;28:354–356.
8. Brannan S, Browne B, Clark BJ. Massive infarction of ocular tissues complicating a necrotic uveal melanoma. Eye (Lond) 1998; 12: 324–325.
9. Bhagat S, Ramaesh K, Wharton SB, Dhillon B. Spontaneous acute scleritis and scleral necrosis in choroidal malignant melanoma. Eye (Lond) 1999; 793–795.
10. Tabassian A, Zuravieff JJ: Necrotic choroidal melanoma with orbital inflammation. Arch Ophthalmol 1995;113:1576–1577.
11. Thareja S, Rashid A, Grossniklaus HE. Spontaneous necrosis of choroidal melanoma. Ocular Oncology and Pathology 2015;1:1:63-9.
12. Peddada K, Dalvin LA, Mashayekhi A, Shields CL. Diagnostic challenges in necrotic uveal melanoma. Retinal Cases & Brief Reports. 2019 Aug [epub ahead of print].
13. Egeland TA, Gaustad JV, Galappathi K, Rofstad EK. Magnetic resonance imaging of tumor necrosis. Acta Oncologica (Stockholm) 2011;50:427–434.
14. Sellam A, Desjardins L, Barnhill R, et al. Fine needle aspiration biopsy in uveal melanoma: technique, complications, and outcomes. Am J Ophthalmol 2016;162:28–34.



